(UroToday.com) The 2022 GU ASCO Annual meeting included a prostate cancer session highlighting work from Dr. Saro Kasparian and colleagues presenting results of real-world experience using the oral gonadotropin-releasing hormone (GnRH) antagonist relugolix. The GnRH analog leuprolide often requires concomitant therapy with an androgen receptor antagonist to bypass testosterone flare. GnRH antagonists were developed in part to circumvent this flare and to achieve faster and more consistent testosterone suppression. Relugolix was given FDA approval for use in prostate cancer based on the phase 3 HERO trial.1 but some real-world concerns include compliance, affordability, and performance in patients transitioning from another GnRH agent or in combination with other therapeutic agents. As such, the aim of this study was to evaluate the real-world implications of prescribing and/or switching to relugolix.
This was a single institution retrospective study conducted between January 2021 and August 2021 on patients prescribed relugolix. Treatment data, including concomitant therapeutic agents, were collected. Compliance data was measured via chart review and pharmacy dispensary records. Patients were classified as either newly castrated or transitioning from another agent. PSA and testosterone levels were tabulated, as were reported adverse effects, and reasons for discontinuation (if applicable) including financial toxicity were noted.
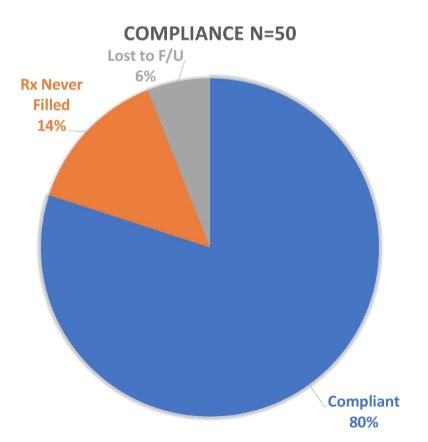
There were 50 patients included in this study: 15 (30%) treated in an adjuvant setting, 18 (36%) for biochemical recurrence, and 17 (34%) for metastatic prostate cancer. There were 12% of patients on concomitant therapy with abiraterone, 4% with enzalutamide, and 2% with apalutamide. Overall, 80% reported compliance to relugolix, and 7 (14%) patients never filled their prescription:
There were 30 (60%) patients that were newly castrated (castration restart or naïve) while 20 (40%) were transitioned from another GnRH medication:
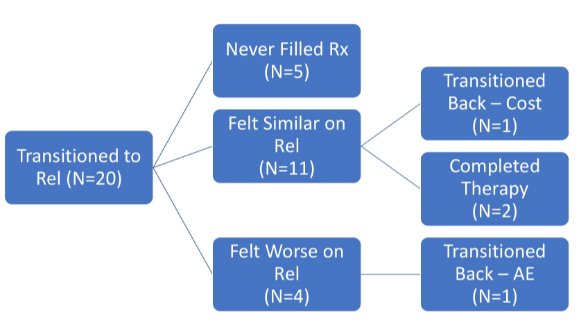
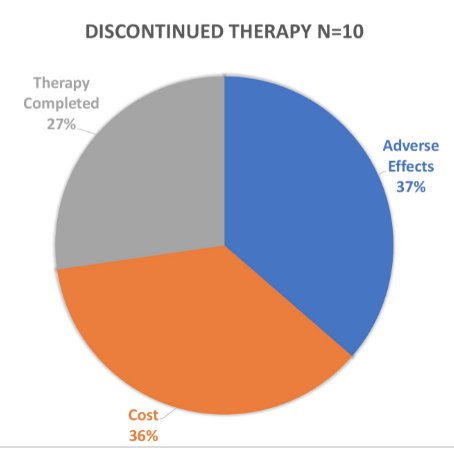
No changes in PSA or testosterone were noted in patients switched from injected GnRH to relugolix. The most common documented adverse effects included hot flashes (24%), fatigue, (29%), and weight gain (9%); no unexpected toxicity was reported in combination with abiraterone. Among the 10 patients who discontinued therapy, 36% did so due to cost, 37% due to adverse effects, and 27% due to therapy completion:
Four of 16 (25%) patients who transitioned from injection GnRH felt symptoms were worse on relugolix.
Dr. Kasparian concluded this analysis of real-world experience with relugolix with the following take-home messages:- In early experience with relugolix, patients did comply with therapy based on self-reporting, pharmacy fill dates, and laboratory evidence of castration
- However, financial toxicity was and remains a significant barrier both accessing relugolix and remaining on relugolix therapy
- While a differential toxicity was reported by those who switched from injection GnRH, sometimes leading to discontinuation, there were no new safety signals reported, especially in combination with abiraterone
Presented by: Saro Kasparian, MD, City of Hope Comprehensive Cancer Center, Duarte, CA
Co-Authors: Oren Wei, Christopher Lehmer, Sumanta K. Pal, Yung Lyou, Tanya B. Dorff
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, Thursday Feb 17 – Saturday Feb 19, 2022
References:


