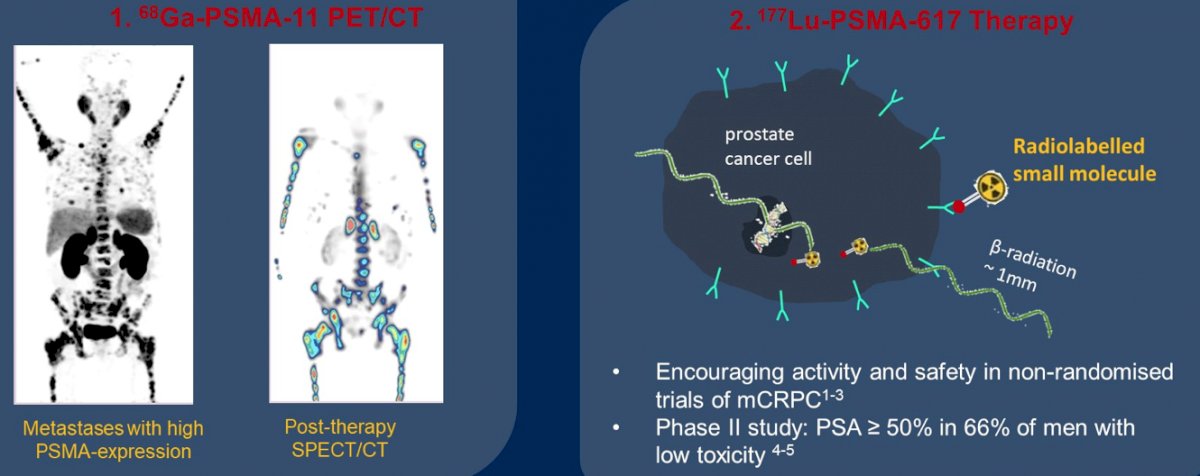
(UroToday.com) The 2022 GU ASCO Annual meeting included a session on novel treatment implementation focusing on PSMA targeting and beyond, with a presentation by Dr. Shahneen Sandhu discussing targeting prostate-specific membrane antigen (PSMA), specifically lutetium and the next wave of novel radiopharmaceuticals. Dr. Sandhu notes that PSMA is a glutamate carboxypeptidase II transmembrane protein that has a pro-proliferative function by activating PI3K and AKT/mTOR pathways via glutamate cleavage. High cellular PSMA expression is associated with poorer patient outcomes and is prevalent in castration resistance. PSMA theranostics combines the diagnostic companion of 68Ga-PSMA-11 PET/CT with the targeted therapeutic of 177Lu-PSMA-617 therapy:
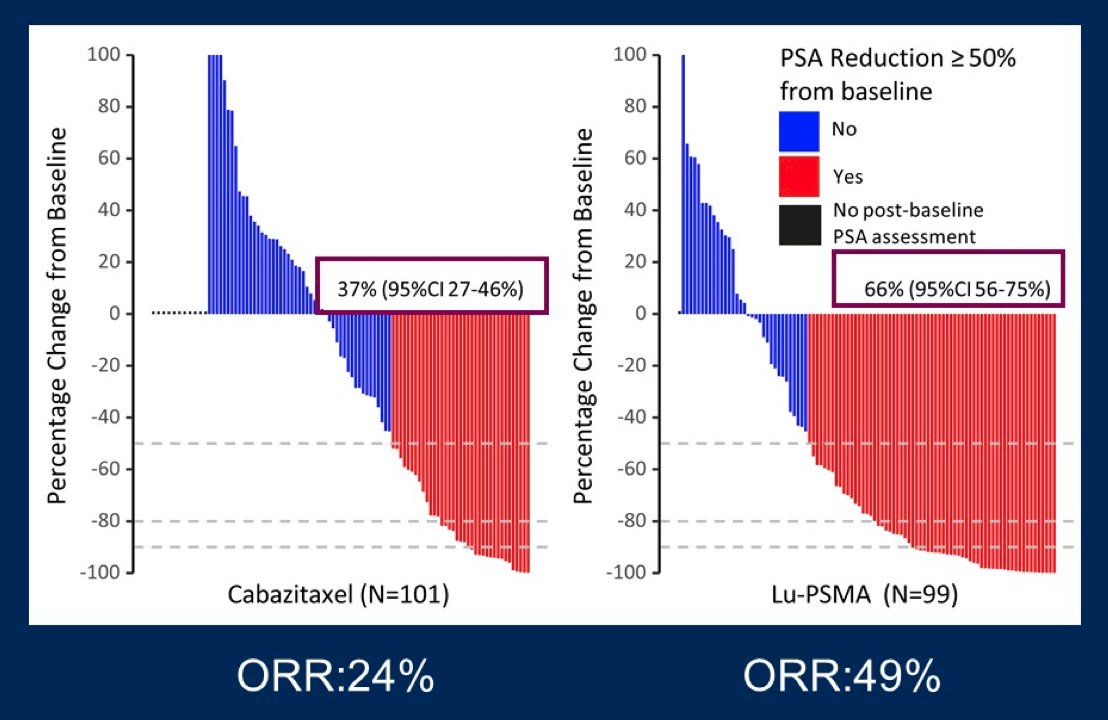
Dr. Sandhu then discussed the TheraP trial, which was an ANZUP/PCFA sponsored, phase III randomized controlled trial of a direct targeted radioligand. TheraP enrolled patients with mCRPC who had previously received docetaxel and were eligible to receive cabazitaxel. Patients were required to have progressive disease with a rising PSA with absolute PSA of 20 ng/mL or higher. All patients underwent both Ga-68-PSMA-PET/CT and F-18-FDG-PET/CT prior to randomization. To be eligible for inclusion, patients must have had a high avidity lesion on PSMA PET/CT (SUV max >20 at any site) with measurable disease with SUV max of 10 or greater. Further, there could be no sites of disease which were FDG positive but PSMA negative. Among 200 men, randomization was performed in a 1:1 fashion to 177Lu-PSMA-617 or cabazitaxel. The primary study outcome was PSA response and was operationalized looking at a response of at least 50% from baseline. Compared to those receiving cabazitaxel (37%, 95% CI 27 to 46%), responses were significantly higher among those who received Lu-PSMA (66%, 95% CI 56 to 75%) with an absolute difference of 29% (95% CI 16 to 42%, p<0.0001):
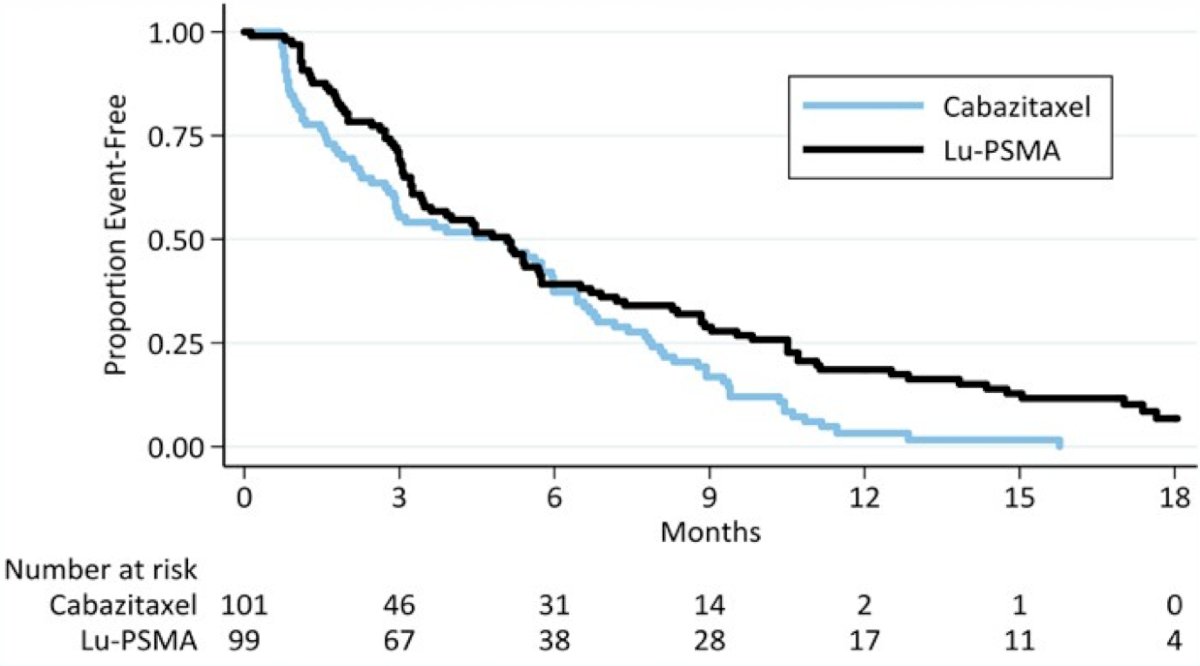
Over a median follow-up of 18.4 months, PFS was significantly longer in those assigned 177Lu-PSMA-617 rather than cabazitaxel (rates at 1 year 19% [95% CI 12-27%] versus 3% [1-9%], HR 0.63, 95%CI 0.46-0.86; p = 0.0028) based on 173 events.
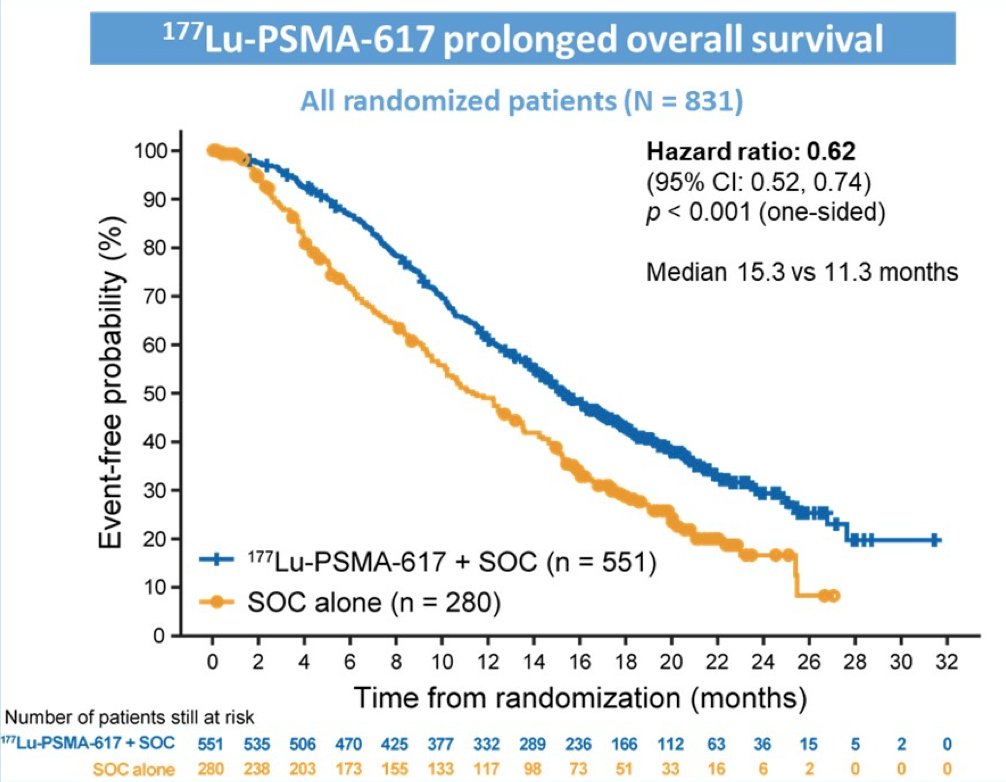
The VISION trial was an international, randomized, open-label phase III study evaluating 177Lu-PSMA-617 in men with PSMA-positive metastatic castration-resistant prostate cancer who had previously received treatment with next-generation androgen receptor signaling inhibition (abiraterone, enzalutamide, etc) and one or two prior lines of taxane chemotherapy [2]. Following enrollment, patients were randomized in a 2:1 fashion to receive either 177Lu-PSMA-617 (7.4 GBq every 6 weeks x 6 cycles) plus standard of care or standard of care alone. Among 1,179 screened patients, the VISION trial enrolled 831 patients, including 551 patients were allocated to 177Lu-PSMA-617 + standard of care and 280 were allocated to standard of care only. Over a median study follow-up of 20.9 months, treatment with 177Lu-PSMA-617+ standard of care significantly improved overall survival by a median of 4.0 months (median overall survival, 15.3 vs 11.3 months; HR, 0.62 [95% confidence interval 0.52, 0.74]; p < 0.001, one-sided), compared to standard of care alone, in the overall cohort of all randomized patients (n=831):
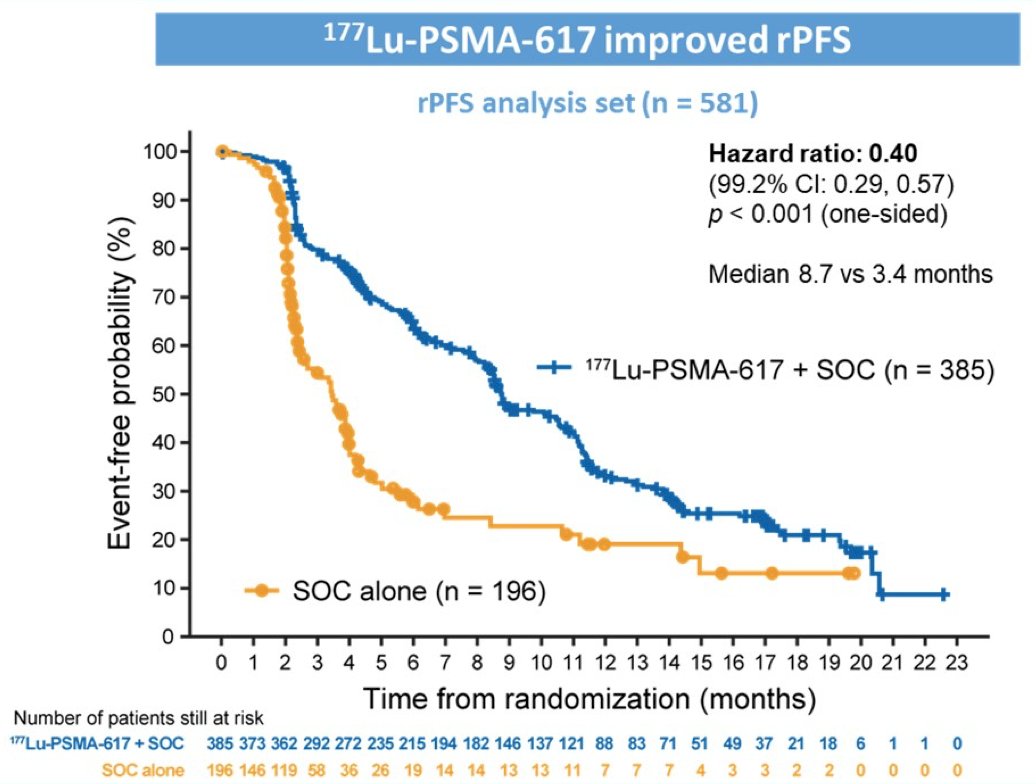
The second alternate primary endpoint showed that treatment with 177Lu-PSMA-617 + standard of care significantly improved radiographic progression free survival by a median 5.3 months (median radiographic progression free survival, 8.7 vs 3.4 months; HR, 0.40 [99.2% confidence interval: 0.29, 0.57]; p < 0.001, one-sided):
Dr. Sandhu highlighted that, based on the forest plot assessing the subgroup analyses for VISION, there was a benefit for 177Lu-PSMA-617 + standard of care for both patients planning to receive an androgen receptor pathway inhibitor (HR 0.54, 95% CI 0.41-0.70) and those not planning to receive an androgen receptor pathway inhibitor (HR 0.68, 95% CI 0.53-0.87). One issue with 177Lu-PSMA-617 is that although we can target PSMA positive patients with radioligand treatment, those that are PSMA negative and FDG positive cannot be targeted, and these are the patients with the most aggressive disease. Dr. Sandhu emphasized that there are several ways for improving responses to 177Lu-PSMA-617 therapy, many of which can be manipulated and improved, including:
- Number of receptors expressed on the cancer cell surface
- Heterogeneity of the targeted receptor
- Radiation dose and fractionation to the cancer cell tumor size matters
- Power and range of internalized radioactive decay
- Radiation sensitivity of the cancer cell
- Unknown mechanisms
Advancing theranostics may have to rely on immunotherapy, upregulation (with apalutamide, abiraterone, and enzalutamide), and radiosensitization (with chemotherapy and PARP inhibitors). As such, the ENZA-p trial is a randomized phase II trial using PSMA as a therapeutic agent and prognostic indicator in men with mCRPC treated with enzalutamide. The trial design for ENZA-p is as follows:
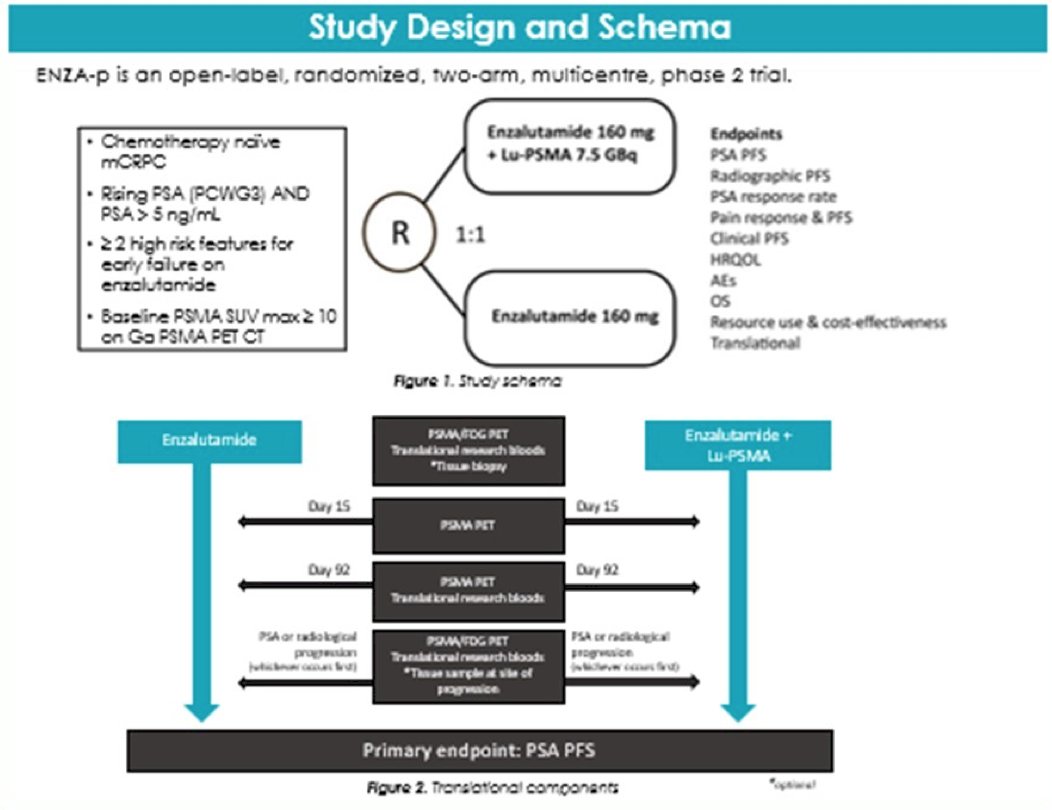
The rationale for this combination is that enzalutamide may lead to a phenotype adjustment, thus potentially providing increased sensitivity to 177Lu-PSMA-617 radioligand therapy.
Cell death from radiation is secondary to apoptosis, mitotic catastrophe, autophagic cell death, necroptosis, or necrosis. The type of cell death induced is dependent on tumor genetics, the tumor microenvironment, and radiation dose and schedule. Furthermore, immune response is integral to translating DNA damage to cell death, particularly at lower radiation doses. 177Lu-PSMA-617 induces predominantly DNA single strand breaks, whereas PARP-1 facilitates single-strand break repair by activating and engaging repair enzymes. PARP inhibitors function by increasing translation of single-strand breaks to double-strand breaks with 177Lu-PSMA-617, which is being tested in the ongoing LuPARP trial.
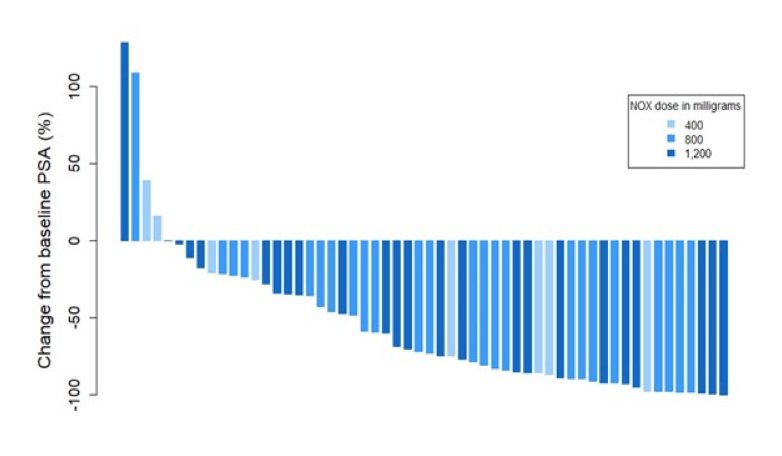
Dr. Sandhu then discussing the LuPIN trial, a phase I dose-escalation/expansion trial of a radiation sensitizer (NOX66) + 177Lu-PSMA-617 (7.5 Gbq). Among 56 men (75% high volume > 20 metastases) with mCRPC treated with 2 taxanes plus an androgen receptor inhibitor, the PSA50 response rate was 61%, PSA PFS was 7.5 months (95% CI 5.9-9.0), and overall survival was 19.7 months (95% CI 9.5-30). As follows is the waterfall plot for change from baseline PSA:
There are several trials that are targeting PSMA and immune checkpoint inhibitors, including:
- PRINCE: 177Lu-PSMA-617 + pembrolizumab
- NCT03805594: 177Lu-PSMA-617 + pembrolizumab
- Phase I/II trial of pembrolizumab and androgen receptor signaling inhibitor +/- 225Ac-J591 for mCRPC
- EVOLUTION: 177Lu-PSMA-617 vs 177Lu-PSMA-617 with ipilimumab + nivolumab
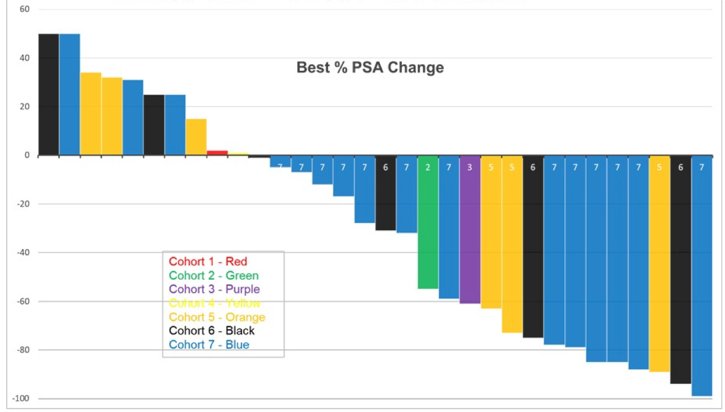
First presented at ASCO 2021, Dr. Scott Tagawa and colleagues presented results of a dose-escalation plus expansion cohort of a first in-human study of 225Ac-J591. There were 32 men treated with a single dose of 225Ac-J591 on 7 dose levels with expansion at the highest dose level (n = 16). One of six patients in cohort 6 (80 KBq/kg) had dose-limiting toxicity (grade 4 anemia and platelets) with 0 of 6 patients at the highest dose level (93.3 KBq/Kg) and thus this dose was expanded. High-grade adverse events were restricted to hematologic: in addition to dose-limiting toxicity, 4 (12.5%) patients with grade 3 platelets and 2 (6.2%) patients with grade 3 neutropenia. Non-hematological adverse events were restricted to grade 1/2 and included: 10 patients (31.2%) with fatigue, 5 patients (15.6%) with pain flare, 14 patients (43.7%) with nausea, 8 patients (25%) with grade 1 xerostomia (of which 5 received prior 177Lu-PSMA), and 12 patients (37.5%) with AST elevation. Despite prior treatment including 177Lu-PSMA and no selection for PSMA expression, 22 (68.8%) patients had any PSA decline, and 14 (43.8%) had > 50% PSA decline:
Dr. Sandhu emphasized that we are currently in a rapidly evolving therapeutic landscape of radiopharmaceutical +/- combination therapy:
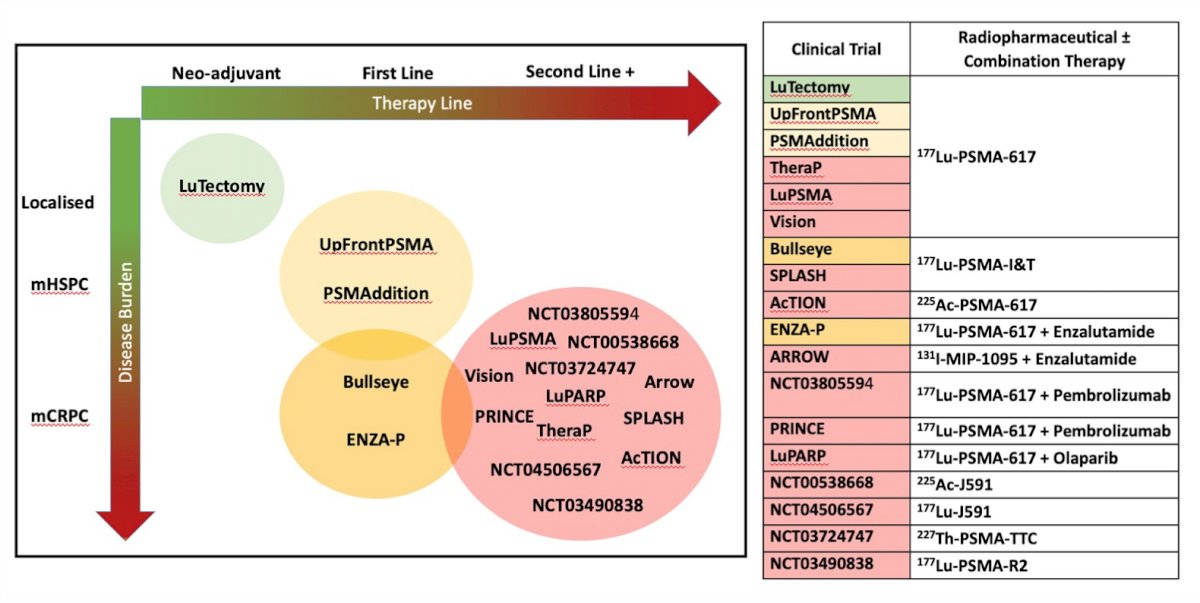
Dr. Sandhu concluded her presentation discussing targeting PSMA, specifically lutetium and the next wave of novel radiopharmaceuticals with the following summary points:
- There are promising early randomized trial results
- We need to optimize dose delivery based on the volume of disease and radiation sensitivity, specifically reimagining dosimetry and radionuclide choice
- We need to address heterogeneity by using PSMA radionuclide therapy as an adjunct treatment with synergistic combinations
- We need to understand the biology of PSMA well to move treatment effectively into an earlier disease space
- Options for enhancing radiation sensitivity include DNA-damage repair inhibitors and immunotherapy (abscopal effect as an adjunctive)
- We must develop better predictive biomarker nomograms for treatment response and combination therapies
Presented by: Shahneen K. Sandhu, Peter MacCallum Cancer Center, Melbourne, Australia
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, Thursday Feb 17 – Saturday Feb 19, 2022
References:
- Hofman MS, Emmett L, Sandhu S, et al. [(177)Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): A randomized, open-label, phase 2 trial. Lancet. 2021 Feb 27;397(10276):797-804.
- Sartor O, de Bono J, Chi KN et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2021 Sep 16;385(12):1091-1103.


