(UroToday.com) On the first day of the American Society for Clinical Oncology (ASCO) Genitourinary Cancer Symposium 2022, Poster Session A focussed on the care of patients with prostate cancer. Dr. Zhang presented a poster describing the use of BXCL701 in patients with metastatic castration-resistant prostate adenocarcinoma cancer (mCRPC). BXCL701, also known as talabostat, is an oral small molecule inhibitor of dipeptidyl peptidases (DPP)—primarily DPP 8/9 & DPP 4— which triggers inflammasome to alert and prime immune cells. This leads to induction of IL-18 & IL-1ß, bridging innate & adaptive immunity.
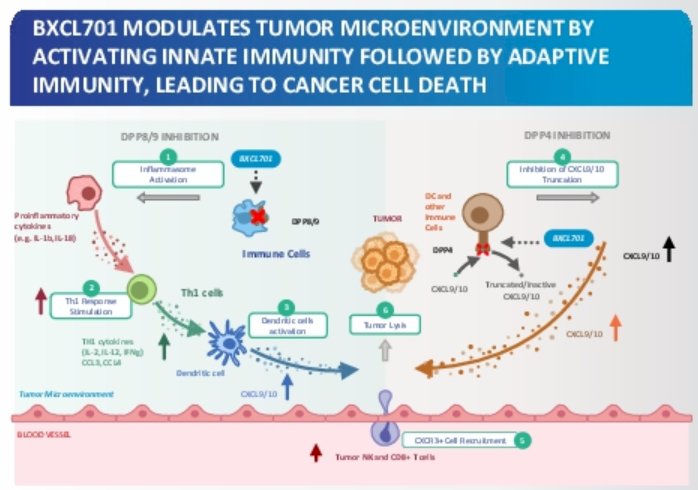
While initial results on the phase 2a adenocarcinoma cohort treated with BXCL701 in combination with pembrolizumab were reported at ESMO 2021, Dr. Zhang presented updated safety and efficacy analyses after enrollment of 40 patients for the adenocarcinoma cohort.
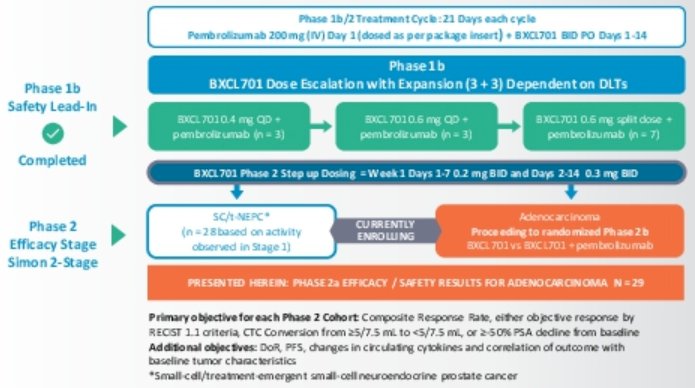
To be eligible for this phase 2a study, patients with metastatic prostate adenocarcinoma were required to have progression by PCWG3 on 1 or 2 androgen signaling inhibitors and ≥1 prior line of taxane chemotherapy. Patients also had to have ECOG performance status of 0-2. Notably, patients were excluded if they had previously received an immune checkpoint inhibitor or more than 2 prior regimes of cytotoxic chemotherapy.
Following enrollment, patients received pembrolizumab (200 mg IV q21-days) and BXCL701 0.2 mg BID for a week with step-up to 0.3 mg BID on days 8-14, and 0.3 mg BID on days 1-14 of subsequent cycles. The primary endpoint of interest is Composite Response, defined as RECIST 1.1 ± PSA50 ± CTC count conversion. The phase 2 component of this study uses a Simon 2-stage minimax design with 15 evaluable patients in stage 1 and 13 in stage 2.
With a data cut-off of November 24, 2021, 42 patients with adenocarcinoma were enrolled of whom 29 were evaluable. All patients had received prior chemotherapy while 17% had received enzalutamide alone, 21% had received abiraterone alone, and 59% had received both novel hormonal agents. In total, patients had received a median of 5 prior lines of therapy (range 1-11).
The median duration of treatment was 10 weeks (range 4 to 59 weeks).

Over a median follow-up duration of 12 weeks (range 1-54), a composite response was seen in 21% of patients with a partial response in 4 patients, stable disease in 11, non-CR/non-PD in 15, and progressive disease in 3. Thus, the overall disease control rate was 83%. CTC response rates were relatively low, in 2 of 11 evaluable patients.

In terms of toxicity, the combination of BXCL701 and pembrolizumab demonstrated acceptable tolerability without evidence of increased immune-related AEs compared to historic controls with checkpoint inhibitors. AEs consistent with cytokine activation were observed (hypotension, oedema, fever, fatigue) though the majority of events were low grade. Serious adverse events were identified in 5 patients.
In conclusion, oral BXCL701 in combination with pembrolizumab demonstrates promising anti-tumor activity with manageable toxicity in very late-line, refractory mCRPC adenocarcinoma. These data form the basis of a trial expansion to include randomization to the bomxination of BXCL701 with pembrolizumab versus BXCL701 alone.Presented by: Jingsong Zhang MD, PhD, Moffitt Cancer Center, Tampa, FL


