(UroToday.com) On the first day of the American Society for Clinical Oncology (ASCO) Genitourinary Cancer Symposium 2022, Poster Session A focused on the care of patients with prostate cancer. Dr. Vaishampayan presented a poster examining ModraDoc006, a novel, oral tablet formulation of docetaxel. In its intravenous formulation, docetaxel and oral prednisone is a standard of care regimen in both metastatic hormone-sensitive prostate cancer and metastatic castration-resistant prostate cancer (mCRPC). ModraDoc006 is a novel, oral tablet formulation of docetaxel which, to enhance the bioavailability, is co-administered with ritonavir (/r), an inhibitor of cytochrome p450 3A4 and P-glycoprotein metabolic enzymes. This oral combination therapy, dubbed ModraDoc006/r, is postulated to offer a number of advantages to intravenous docetaxel on the basis of safety profile, avoidance of infusions, patient quality of life (QoL), and overall resource utilization.
To assess this, the authors performed an open label 1:1 randomized study of ModraDoc006/r bi-daily weekly dosing (BIDW) regimen versus IV docetaxel 75 mg/m2 in 21-day cycles.
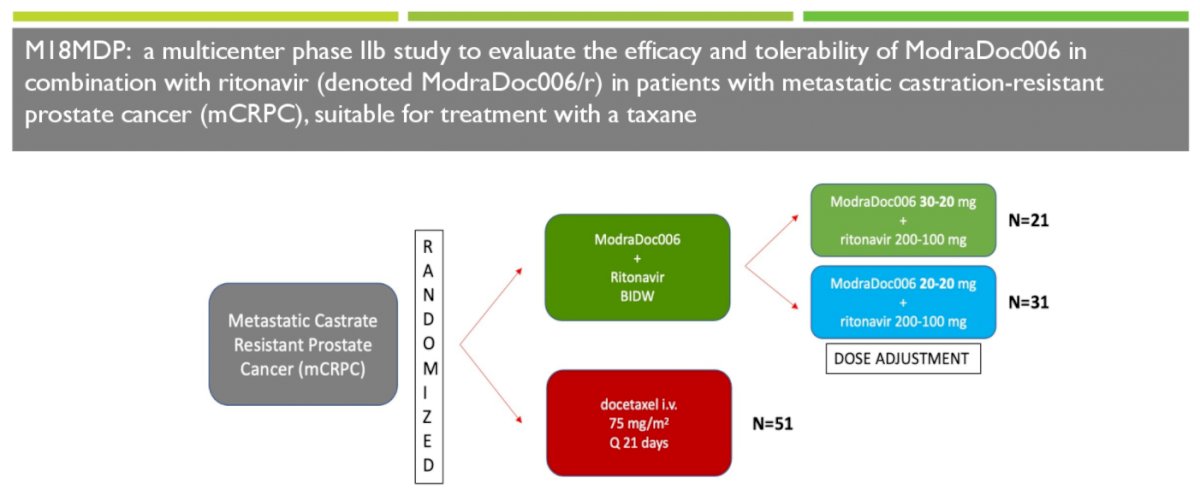
The authors initially employed BIDW dosing of 30-20 mg ModraDoc006 combined with 200-100 mg ritonavir, administered on days 1, 8 and 15 of a 21-day cycle. After 39 patients the starting dose was reduced to 20-20 mg BIDW with ritonavir 200 mg in the morning and 100 mg with the evening dose to improve tolerability. Throughout the entire trial, all patients received 5 mg oral prednisone twice daily. The authors assessed the primary endpoint of radiographic progression free survival (rPFS) per PCWG-3 criteria, with key secondary endpoints including ORR, PSA-PFS, time to skeletal-related events, disease control rate, duration of response, and safety assessments. Further, they assessed patient-reported outcomes and health-related QoL using treatment satisfaction and FACT-P questionnaires at baseline and after cycles 3, 6 and 10.
The authors successfully completed accrual of 103 patients of whom 51 were randomized to intravenous docetaxel and 52 to ModraDoc006/r though only 49 patients randomized to docetaxel received treatment and were included in the safety population. Among those randomized to ModraDoc006/r, 21 were randomized to the 30-20 mg treatment regime and 31 received ModraDoc006/r 20-20 mg following dose adjustment. At baseline, median PSA was somewhat lower among those randomized to ModraDoc006/r (50.6 ng/mL, range 0-1181) compared to intravenous docetaxel (81 ng/mL, range 1-1460). Between 40-50% of patients had previously receive enzalutamide or abiraterone.
In terms of safety data, ModraDoc006/r at 20-20/200-100mg doses appears to have fewer, less severe toxicity. Notably, all graded neutropenia and neuropathy was noted in 0% and 6% with ModraDoc006/r 20-20 mg therapy which is lower than the rates seen with ModraDoc006/r 30-20 mg (14% and 14%), or intravenous docetaxel (27% and 31%). Additionally, alopecia was also reduced at 23% on ModraDoc006/r 20-20 mg and 29% on ModraDoc006/r 30-20 mg vs 43% on intravenous docetaxel. However, GI toxicities were slightly more frequent, though these were predominantly mild, in the ModraDoc006/r arm: at 20-20 mg dose, all grades diarrhea 32% (3% ≥G3), nausea 29% and stomatitis 3% (G3); at 30-20 mg dose 62% diarrhea (19% ≥G3), 38% nausea and 14% stomatitis (5% ≥G3); and in IV docetaxel, 24% diarrhea, 16% nausea and 10% stomatitis (4% ≥G3).
In terms of oncologic efficacy, overall response rates were comparable between intravenous docetaxel (38.7%, 95% CI 21.8 to 57.8%) and ModraDoc006/r (44.1%, 95% CI 27.2 to 62.1%, p=0.80). PSA response rates were similarly comparable (56.5% vs 50.0%, p=0.68).

Thus, the authors conclude that this trial demonstrated that ModraDoc006/r has convenient oral weekly dosing with a better toxicity profile and comparable oncologic efficacy.
Presented by: Ulka N. Vaishampayan MD, University of Michigan Cancer Center, Detroit, MI


