(UroToday.com) On the first day of the American Society for Clinical Oncology (ASCO) Genitourinary Cancer Symposium 2022, Poster Session A focused on the care of patients with prostate cancer. Dr. Spohn presented a poster highlighting the potential for positron emission tomography targeting prostate specific membrane antigen (PSMA-PET) to allow focal dose-escalated radiotherapy of primary prostate cancer. The use of PSMA-PET has allowed substantial changes in the care of patients with prostate cancer, particularly in terms of earlier detection of small volume metastatic disease. However, it may also allow improved focal dose escalation in radiotherapy (RT) due to superior coverage of intraprostatic tumour burden.
To assess this, the authors performed a 2-armed, non-randomized HypoFocal phase II trial to investigate the safety of implementation of PSMA-PET-based focal therapy planning in external beam radiotherapy (EBRT) and high-dose-rate brachytherapy (HDR-BT). To do so, they enrolled patients with intermediate- and high risk prostate cancer with cN0 and cM0 disease, staged by mpMRI and PSMA PET. Patients then received EBRT in 20 fractions with 60 Gy to the prostate. Patients in arm A further received up to 75 Gy to mpMRI- and PSMA-PET defined boost volumes whereas those in arm B received HDR-BT with 15 Gy to the prostate and a boost of up to 19 Gy, followed by EBRT with 44Gy in 20 fractions. 36% of patients received androgen deprivation therapy. While the overall study seeks to assess volumes, treatment plans, gastrointestinal (GI) and genitourinary (GU) toxicities according to CTCAE v5.0, and Quality of Life (QoL), this presentation focused on the planned safety analysis after 6 months of follow-up (FU).
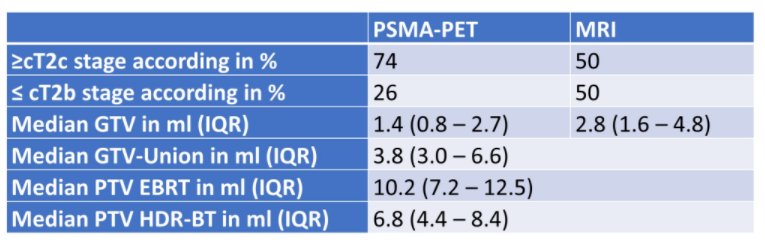
To date, 25 patients were enrolled in each study arm in two centers (Freiburg and Berlin). The use of PSMA-PET results in significant clinical upstaging compared to mpMRI (p=0.007). GTV-PET and GTV-Union were significantly larger than GTV-MRI, resulting in large boost volumes (see table). Boost volumes received a median mean dose of 70 Gy in EBRT and a median D90 of 19 Gy in HDR-BT.
At 6 months FU, the prevalence of ≥ grade 2 GU and GI toxicity was 4% and 0% for arm A and 12 and 4% for arm B.

Two patients experienced grade 3 GI toxicity 9 and 12 months after radiotherapy, related to their post-treatment biopsy. No signification change in quality of life was observed after 6 months of follow-up.

Thus, the authors conclude that use of PSMA-PET in primary prostate cancer significantly alters clinical staging compared to mpMRI and as a result affects RT treatment planning. However, boost delivery was feasible and relatively well tolerated.
Presented by: Simon Spohn, MD, Department of Radiation Oncology, Medical Center – the University of Freiburg, Faculty of Medicine

