(UroToday.com) On the first day of the American Society for Clinical Oncology (ASCO) Genitourinary Cancer Symposium 2022, Trials in Progress Poster Session A focussed on ongoing trials that will contribute to the care of patients with prostate cancer moving forward. Dr. Sartor described the rationale and design of the PSMAfore trial.
[177Lu]Lu-PSMA-617 (177Lu-PSMA-617) is a high-affinity prostate-specific membrane antigen (PSMA)-targeted radioligand therapy. 177Lu-PSMA-617 delivers β-particle radiation to PSMA-expressing cells and their surrounding microenvironment. In the phase 2 TheraP and phase 3 VISION trials, 177Lu-PSMA-617 significantly prolonged radiographic progression-free survival (rPFS) and overall survival (OS) in patients with metastatic castration-resistant prostate cancer (mCRPC) who have received a number of previous lines of therapy including androgen receptor pathway inhibitors (ARPI) and taxanes. Moving forward, there is an outstanding question as to the role of 177Lu-PSMA-617 earlier in the treatment trajectory. Thus, PSMAfore is investigating the effect of 177Lu-PSMA-617 or a change in ARPI on rPFS taxane-naïve patients with mCRPC.
To address this, PSMAfore (NCT04689828) is a multicenter, open-label, randomized phase 3 trial in adult men with progressive mCRPC and confirmed PSMA expression by [68Ga]Ga-PSMA-11 PET/CT. Eligible patients must be taxane-naïve in the metastatic setting and have received one prior ARPI and be a candidate for a change in ARPI. Additionally, patients must have an Eastern Cooperative Oncology Group performance status of 0 or 1; a castrate level of serum/plasma testosterone ( < 50 ng/dL or < 1.7 nmol/L); and no ongoing toxicity of grade 3 or greater related to prior therapies. The authors aim to recruit and randomize 450 patients in a 1:1 fashion to receive 177Lu-PSMA-617 (7.4 GBq i.v. every 6 weeks for 6 cycles) or a change in ARPI to either abiraterone or enzalutamide. In both arms, best supportive care is allowed.
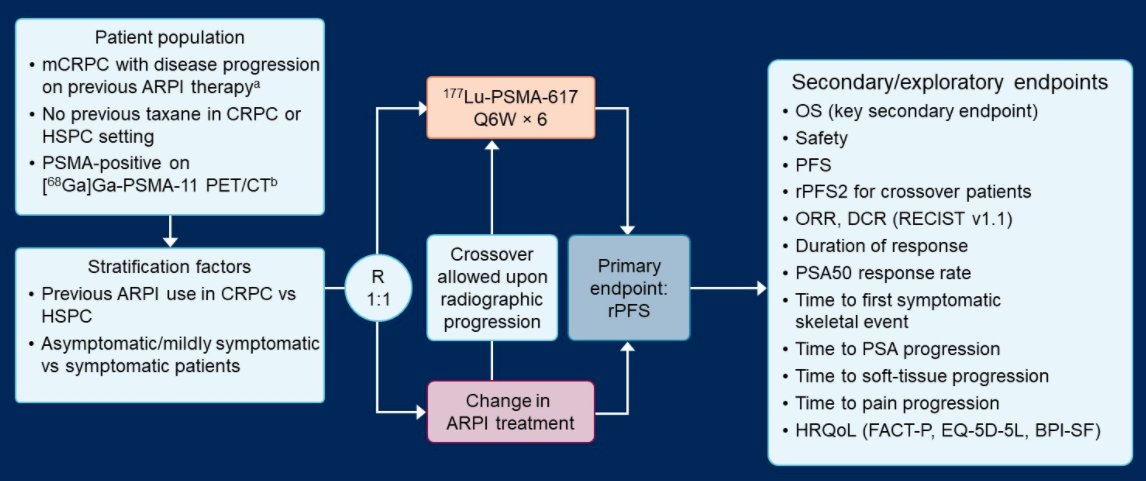
Stratification factors are prior ARPI use in castration-resistant vs hormone-sensitive prostate cancer settings and pain symptomatology. The primary endpoint is radiographic progression free survival (rPFS) according to PCWG3-modified RECIST v1.1 criteria. There are a large number of other secondary and exploratory endpoints, including the key secondary endpoint of overall survival.
Participants with blinded independent centrally confirmed radiographic progression in the ARPI arm can crossover to the 177Lu-PSMA-617 arm. The primary efficacy and safety analyses are planned with a sample of 156 rPFS events which will allow for a 95% power to detect a hazard ratio of 0.56 for rPFS with an overall one-sided significance level of 0.025. Overall survival will be further analysed once approximately 297 deaths have been observed.
The first participant was enrolled on study on June 15, 2021 and patients are currently being recruited in a number of countries in North American and Europe. The estimated study completion date is December 2024.
Presented by: A. Oliver Sartor MD, School of Medicine, Tulane Medical School, New Orleans, LA


