(UroToday.com) In a session on the third day of the American Society for Clinical Oncology (ASCO) Genitourinary Cancer Symposium 2022 entitled Regulating the Wild West: PET-Based Imaging in Trials and the Clinic, Dr. Alicia Morgans presented on the rapid evolution of the prostate cancer treatment landscape. Highlighting the example of a fictious SuperDrug being investigated in the adjuvant setting following prostatectomy for high-risk localized disease, she emphasized that there have been dramatic recent changes in imaging which change how we approach this patient and their care.

One of the more profound advances in the past few years in prostate cancer management has been the widespread adoption of molecularly-targeted PET imaging. Dr. Morgans highlighted data from the proPSMA trial which examined 68Ga-PSMA-11 PET/CT compared to conventional CT scan and bone scan as staging for men with high-risk prostate cancer prior to planned definitive, curative-intent treatment. PSMA imaging was associated with both higher sensitivity (85 vs 38%) and higher specificity (98 vs 91%), resulting in significantly improved accuracy 992 vs 65%, p<0.0001). Further, when examining in the biochemical recurrence setting in the CONDOR trial, PSMA-PET/CT showed high rates of clinical localization, even at very low PSA-levels (down to <0.5 ng/mL) which resulted in a change in planned management in the majority of patients.
Dr. Morgans then highlighted that, in the National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines for Prostate Cancer, staging investigations are warranted for patients with unfavourable intermediate-risk, high-risk, and very-high-risk diseases. While this has been conventional imaging up until recently, as of the third version of the 2022 guidelines, the guideline panel recommends that 68Ga- or F-18 pifluofolastat may be used instead. Further
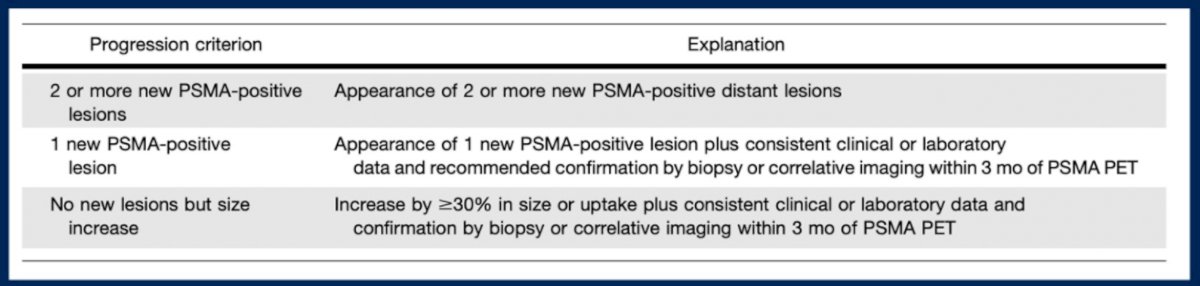
Beyond these initial staging and disease localization in biochemical recurrence indications, PSMA-PET may be used in patients who are undergoing systemic therapy. However, while RECIST criteria of response are well-established, the way to assess serial PSMA-PET images is somewhat less clear. Dr. Morgans highlighted a recent publication in the Journal of Nuclear Medicine which proposed PSMA-PET Progression criteria for patients on systemic therapy. As highlighted in the Table below, this proposal advocates for three progression criteria included 2 or more new PSMA-positive lesions, one new PSMA-positive lesion (requiring correlative support), or an increase in size of existing lesions.
Coming back to the circumstance with which she started her presentation, Dr. Morgan emphasized that the increasing utilization of PSMA-PET imaging will have substantial effects on enrollment of adjuvant trials. First, there would be a substantial stage migration with patients characterized as localized on conventional having visible disease on PSMA-PET. Thus, we must consider if these patients with metastatic disease on PET imaging should be included. Further, if we include them, how should we manage the heterogeneity introduced? Additionally, should PSMA-targeted metastasis directed therapy be allowed prior to “adjuvant” therapy? Finally, in patients with evidence of metastatic disease (by PET only and not conventional imaging), is it reasonable to randomize to ADT alone given the large number of agents with proven survival benefits in metastatic disease which have become standard of care?
Beyond these enrollment considerations, Dr. Morgans highlighted that the use of PET-imaging should make use reconsider what we use as reliable endpoints. First, we must consider whether we want to rely on conventional imaging or PSMA-PET imaging for outcome ascertainment and the detection of progression. If we are to rely on PSMA-PET, we need accepted definitions of progression. Further, we must decide what triggers a change in therapy – progression on PSMA-PET or whether progression must be demonstrated on conventional imaging. Additionally, is the use of metastasis-directed therapy a meaningful progression endpoint? Finally, for those who progress by PET-imaging, but not conventional imaging, should we be mandating systemic therapy for metastatic disease?
Moving from these questions of imaging and disease detection, she highlighted recent treatment advances, including the prolongation of metastasis-free survival in non-metastatic castration resistant disease with the use of apalutamide, enzalutamide, and darolutamide in the SPARTAN, PROSPER, and ARAMIS trials, respectively. In each of these trial, MFS was defined based on conventional imaging. On the basis of these data, for patients with PSA doubling times less than or equal to 10 months, treatment with one of these agents is a category 1 recommendation in the NCCN Clinical Practice guidelines.
Another major recent development, beyond the earlier use of intensified systemic therapy, is the use of metastasis directed therapy. While there is great interest in this approach and many clinicians are employing it, Dr. Morgans highlighted that we don’t have clear evidence that we are affecting the disease trajectory. In prostate cancer, metastasis-directed therapy certainly can delay the initiation of ADT, however.
Dr. Morgans emphasized that, for adjuvant trials, intervening therapies can introduce heterogeneity. For patients who have a rising PSA in this setting, there are a number of potential treatment approaches which may affect long-term outcomes including ADT alone, metastasis directed therapy alone, or a combination of the two (with or without further systemic therapy intensification). Moving forward, trials in the neoadjuvant, adjuvant, and biochemical recurrence settings in prostate cancer will face significant challenges as a result of the evolving landscape of prostate cancer management. These changes will affect trial eligibility, endpoint definitions, and outcome interpretation. Consensus between clinicians, trialists, regulators, and patients is necessary to chart a path forward.
Presented by: Alicia K. Morgans, MD, MPH, Dana-Farber Cancer Institute, Harvard Medical School


