(UroToday.com) The 2022 GU ASCO Annual meeting included a session on the optimization management of localized prostate cancer, specifically looking at artificial intelligence (AI), active surveillance, and intervention, featuring a presentation by Dr. Mary-Ellen Taplin discussing combined modality therapy in localized prostate cancer. Dr. Taplin notes that high-risk localized prostate cancer is associated with an increased risk of morality as highlighted in the following table emphasizing 15-year prostate cancer specific mortality rates:

Currently, there are several paradigms of treatment for high-risk prostate cancer:

However, there is still some question as to the utility of neoadjuvant treatment for these high-risk patients. Interestingly, neoadjuvant treatment is standard of care for breast, rectal, bladder, and other cancers given the improved long-term survival. Neoadjuvant therapy may down-stage local disease, which may facilitate surgical resection and potentially reduce or delay post-surgery treatment. Historical neoadjuvant trials investigated LHRH agonists +/- first generation anti-androgens, but with the majority of patients having low-risk disease. These trials did not systematically evaluate pathologic response and had limited long-term follow-up. More contemporary neoadjuvant trials are investigating more potent androgen targeting agents (abiraterone acetate, enzalutamide, apalutamide), the majority of patients have high-risk disease, there is systematic central pathology review to evaluate response, and long-term follow-up is ongoing.
The optimal response metric in the neoadjuvant setting remains to be fully elucidated, specifically whether we should be conducting short versus long term trials or phase 2 versus phase 3 trials. There may be several potential endpoints in neoadjuvant trials based on the phase they are being conducted:
- Phase 2
- Tissue endpoint: hormone levels, drug levels, or immunohistochemistry
- Pathologic staging endpoint: pathologic complete response, minimal residual disease, volume of residual tumor, cellularity/volume, residual cancer burden
- Intermediate clinical endpoint: PSA/clinical relapse
- Phase 3
- Metastases free survival: ICECaP - rPFS as a surrogate for OS
- MFS + radical prostatectomy pathology: PROTEUS trial (3-year accrual, 4-year follow-up)
Dr. Taplin highlighted that there have been several series of randomized phase 2 trials in the neoadjuvant setting, testing the utility of abiraterone, enzalutamide, and apalutamide. These trials have resulted in the following clinical outcomes:
- pCR rates: 10-13%
- Minimal residual disease rates: 24-30%
- Combined pCR + minimal residual disease rates: 22-40%
- 3-year BCR-free rates: Overall 59.1%; exceptional responder 95.2%; non-responder: 48.7%
Dr. Taplin discussed several important ongoing neoadjuvant trials. First, the Proteus trial is a randomized, double-blind, placebo-controlled, phase 3 study of apalutamide in subjects with high-risk, localized, or locally advanced prostate cancer who are candidates for radical prostatectomy. Patients are randomized to 6 months of apalutamide + ADT versus placebo + ADT followed by radical prostatectomy and 6 months of additional therapy (based on what they received prior to radical prostatectomy) post-operatively. The primary endpoints are pathologic complete response and metastasis free survival, and key secondary endpoints are PSA-free survival and progression free survival. The study schema for Proteus is as follows:
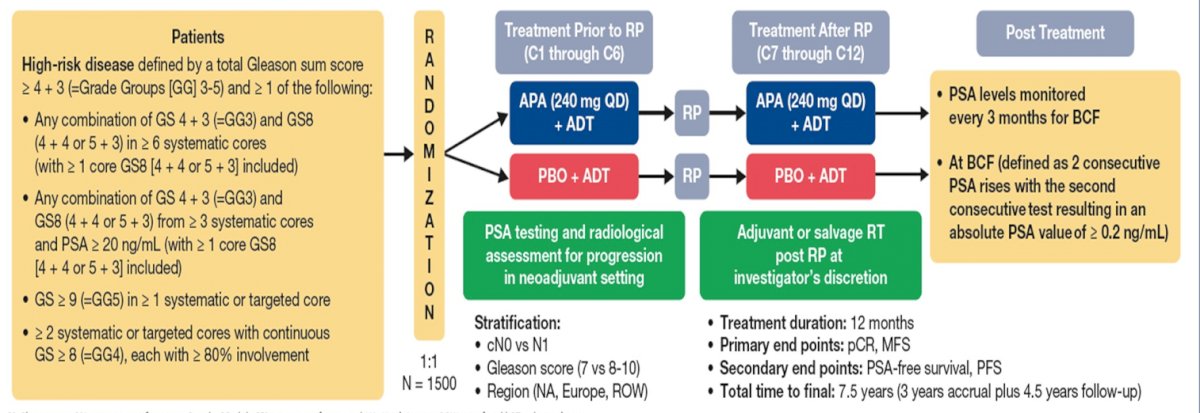
Second, the GUNS trial is a multi-arm, multi-stage adaptive trial in biomarker selected patients with high-risk localized prostate cancer. The primary endpoint of this trial is pathological complete response rate and the trial schema is as follows:

Third, neoadjuvant darolutamide + abemaciclib will be assessed in a phase I lead-in 3+3 design to determine RP2D, followed by a randomized phase 2 study of ADT + darolutamide + abemaciclib versus ADT + darolutamide followed by radical prostatectomy. The primary endpoint is the rate of pathologic complete response + minimal residual disease rate, with the following trial schema:

Dr. Taplin then discussed what’s new in primary radiation for localized high-risk prostate cancer and as salvage therapy after prostatectomy. The RTOG 0815 trial assessed escalated radiotherapy alone or with androgen suppression for intermediate risk prostate cancer. This trial had 1,538 patients with T2b-T2c, Gleason 7, PSA >10-20 prostate cancer that was randomized to dose-escalated radiotherapy alone or combination with LHRH agonist/bicalutamide for 6 months. Radiotherapy was 79.2 Gy external beam radiotherapy or external beam radiotherapy + LDR or HDR brachytherapy boost. Patients were randomized stratified by single versus multiple risk factors, radiotherapy boost, and baseline comorbidity index. This trial found no difference in 5-year overall survival (90% vs 91%), ADT + radiotherapy improving PSA relapse (HR 0.52), distant metastasis (HR 0.25), and prostate cancer specific mortality (HR 0.10). Additionally, grade 3 adverse events were high with ADT + radiotherapy (17.5%) compared to radiotherapy alone (2.3%).
The recently published meta-analysis assessing abiraterone acetate + prednisolone with or without enzalutamide for high-risk non-metastatic prostate cancer from the STAMPEDE platform1 provided additional clarity in this disease space. Eligible patients had high-risk or relapsing with high-risk features non-metastatic prostate cancer; local radiotherapy was mandated for node negative and encouraged for node positive disease. In both trials, patients were randomly assigned (1:1) to ADT alone (control group), or with abiraterone acetate and oral prednisolone. In the second trial with no overlapping controls, the combination-therapy group also received enzalutamide (160 mg daily orally). The primary endpoint of this meta-analysis was metastasis-free survival. Overall, 1,974 patients were randomly assigned to treatment. With a median follow-up of 72 months (IQR 60–84), metastasis-free survival was significantly longer in the combination-therapy groups (median not reached, IQR not evaluable [NE]–NE) than in the control groups (median not reached, IQR 97–NE; HR 0.53, 95% CI 0.44–0.64). Overall survival (median not reached, IQR NE–NE] in the combination-therapy groups vs not reached [IQR 103–NE] in the control groups; HR 0.60, 95% CI 0.48–0.73), prostate cancer-specific survival (median not reached, IQR [NE–NE] vs median not reached [IQRNE–NE]; HR 0.49, 95% CI 0.37–0.65), biochemical failure-free-survival (median not reached, IQR [NE–NE] vs 86 months [IQR 83–NE]; HR 0.39, 95% CI 0.33–0.47), and progression-free-survival (median not reached [IQR NE–NE] vs median not reached [IQR 103–NE]; HR 0.44, 95% CI 0.36–0.54) were also significantly longer in the combination-therapy groups than in the control groups.
Dr. Taplin then discussed several important ongoing trials in this disease space. First, a randomized phase 3 study assessing conventional ADT with or without abiraterone acetate + prednisone and apalutamide following a detectable PSA after radiation and ADT. Of note, inclusion requires any PSA > undetectable after radiation and at least 6, but not more than 8 months of conventional ADT in patients with non-metastatic high-risk or N1 prostate cancer. The trial schema is as follows:
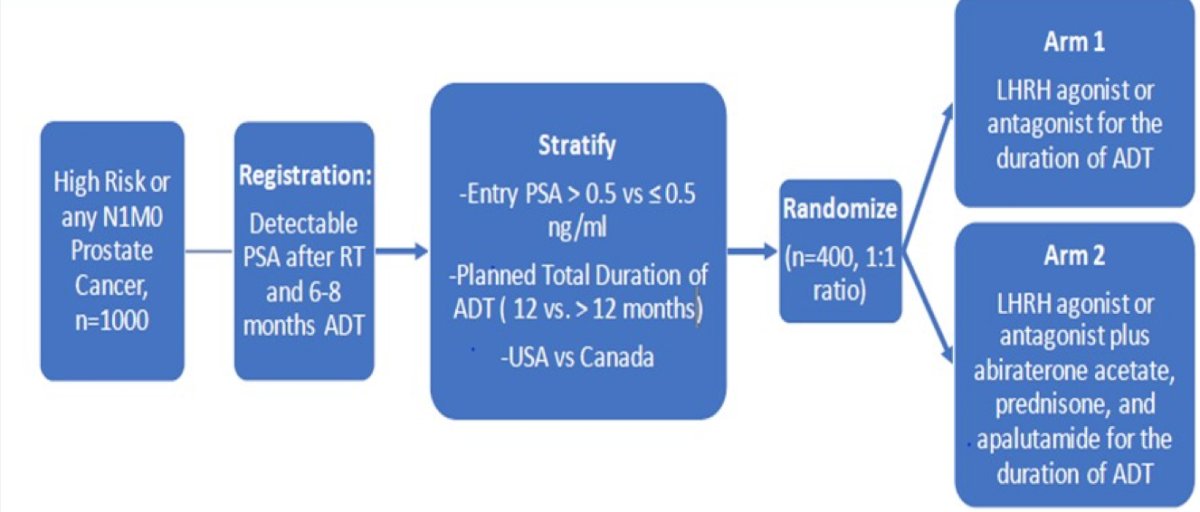
The PREDICT-RT phase 3 randomized trial is for high-risk prostate cancer evaluating de-intensification for lower genomic risk and intensification of concurrent therapy for higher genomic risk with radiation (NRG-GU009). The PI for this trial is Dr. Paul Nguyen and the schema is as follows:

Dr. Taplin concluded her presentation by highlighting several key trials assessing adjuvant systemic therapy after radical prostatectomy. The ERADICATE trial is assessing radiotherapy for Decipher > 0.6, treating these patients with ADT +/- darolutamide. The trial schema for ERADICATE is as follows:

Second, the INNOVATE is for pathologic node positive prostate cancer with a rising PSA, randomizing patients to standard of care + ADT versus standard of care + ADT + apalutamide. Notably, patients must be pN1 with a detectable PSA after radical prostatectomy and prior negative Axumin, F-18, or Ga-68 PSMA PET within 90 days documenting no distant metastatic disease. The primary endpoint is metastasis-free survival. The trial schema for INNOVATE is as follows.

Third, the DASL-HiCaP trial is an ANZUP-led, randomized, phase 3, placebo-controlled, double-blind international trial for men planned for radiotherapy who have very high-risk localized prostate cancer on conventional imaging, or very high-risk features with PSA persistence or rise within one year following radical prostatectomy. The trial is stratified by: radical prostatectomy, use of adjuvant docetaxel, and pelvic nodal involvement. The goal is inclusion of 1,100 participants who will be randomized to darolutamide 600 mg or placebo twice daily for 96 weeks in combination with standard of care: LHRHa for 96 weeks, plus RT starting week 8-24 from randomization.

The primary endpoint is metastasis-free survival (ICECaP-validated), with key secondary endpoints being overall survival, prostate cancer-specific survival, PSA-progression free survival, time to subsequent hormonal therapy, and time to castration-resistance.
Dr. Taplin concluded her presentation discussing combined modality therapy in localized prostate cancer with the following take-home messages
- For multi-modality therapy for patients undergoing prostatectomy for localized high-risk prostate cancer:
- There is currently no phase 3 data to support systemic therapy with radical prostatectomy
- Innovative phase 2 trials are underway with biomarker-directed treatment, including utilization of PSMA-PET and biomarker selection
- The PROTEUS trial will read out in ~2026, with the potential for radical prostatectomy pathology to be a surrogate for metastasis free survival
- More clinical research investment is urgently needed for these patients
- For multi-modality therapy for patients undergoing radiotherapy for localized intermediate and high-risk prostate cancer:
- ADT has value for most patients with intermediate risk prostate cancer undergoing radiation (RTOG 0815)
- Abiraterone acetate/prednisone is recommended in addition to ADT and radiotherapy for localized high-risk prostate cancer undergoing radiation (STAMPEDE). Of note, data from Enza-Rad is expected ~2023-2024
- Trials are ongoing trials evaluating apalutamide and darolutamide with salvage radiotherapy, potentially being an important implication for genomic risk stratification
- Importantly, the burden of increased therapy recommendations needs to be considered, given the added toxicity and cost associated with therapy
Presented by: Mary-Ellen Taplin, MD, Dana-Farber Cancer Institute, Boston, MA
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, Thursday, Feb 17 – Saturday, Feb 19, 2022
References:
- Attard G, Murphy L, Clarke NW, et al. Abiraterone acetate and prednisolone with or without enzalutamide for high-risk non-metastatic prostate cancer: A meta-analysis of primary results from two randomized controlled phase 3 trials of the STAMPEDE platform protocol. Lancet. 23 December 2021 [Epub ahead of print].


