(UroToday.com) The 2022 GU ASCO Annual meeting included a prostate cancer trials in progress session featuring Dr. Rahul Aggarwal presenting a phase 1b study of tarlatamab, a half-life extended bispecific T-cell engager (HLE BiTE immune therapy) targeting DLL3, in de novo or treatment emergent neuroendocrine prostate cancer. Neuroendocrine prostate cancer is an aggressive cancer with no standard treatment approach and poor prognosis. It is usually treatment-emergent, occurring in 15%–20% of patients with metastatic castration-resistant prostate cancer following treatment with androgen signaling inhibitors (ASI) and is characterized by histological transformation from adenocarcinoma to a high-grade neuroendocrine tumor [1]. The tumor associated antigen delta-like ligand 3 (DLL3) has been identified as a promising target in both neuroendocrine prostate cancer and small cell lung cancer (SCLC), as it is highly expressed in these tumors and minimally expressed on normal tissue. Tarlatamab is a DLL3-targeting HLE BiTE® immune therapy designed to bind DLL3 on cancer cells and CD3 on T cells, resulting in T cell activation and expansion and T cell-dependent killing of tumor cells:
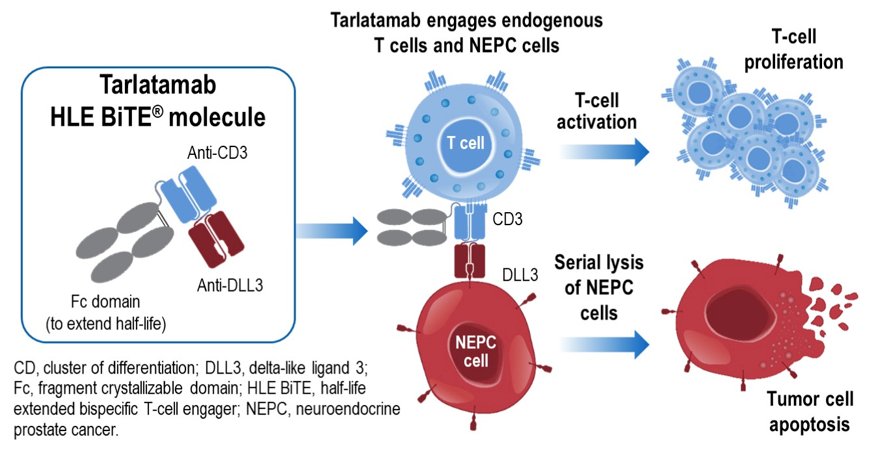
In preclinical studies, tarlatamab induced T-cell dependent lysis of DLL3-expressing neuroendocrine tumor cell lines, including neuroendocrine prostate cancer cells. Interim results of an ongoing first-in-human study in patients with SCLC (NCT03319940) show evidence for tarlatamab efficacy with an acceptable safety profile. Together, these findings support a clinical study of tarlatamab in neuroendocrine prostate cancer.
NCT04702737 is an open-label, phase 1b study evaluating tarlatamab infusion in patients with metastatic de novo or treatment-emergent neuroendocrine prostate cancer, consisting of dose exploration and then dose expansion. The study design of this phase Ib trial is as follows:
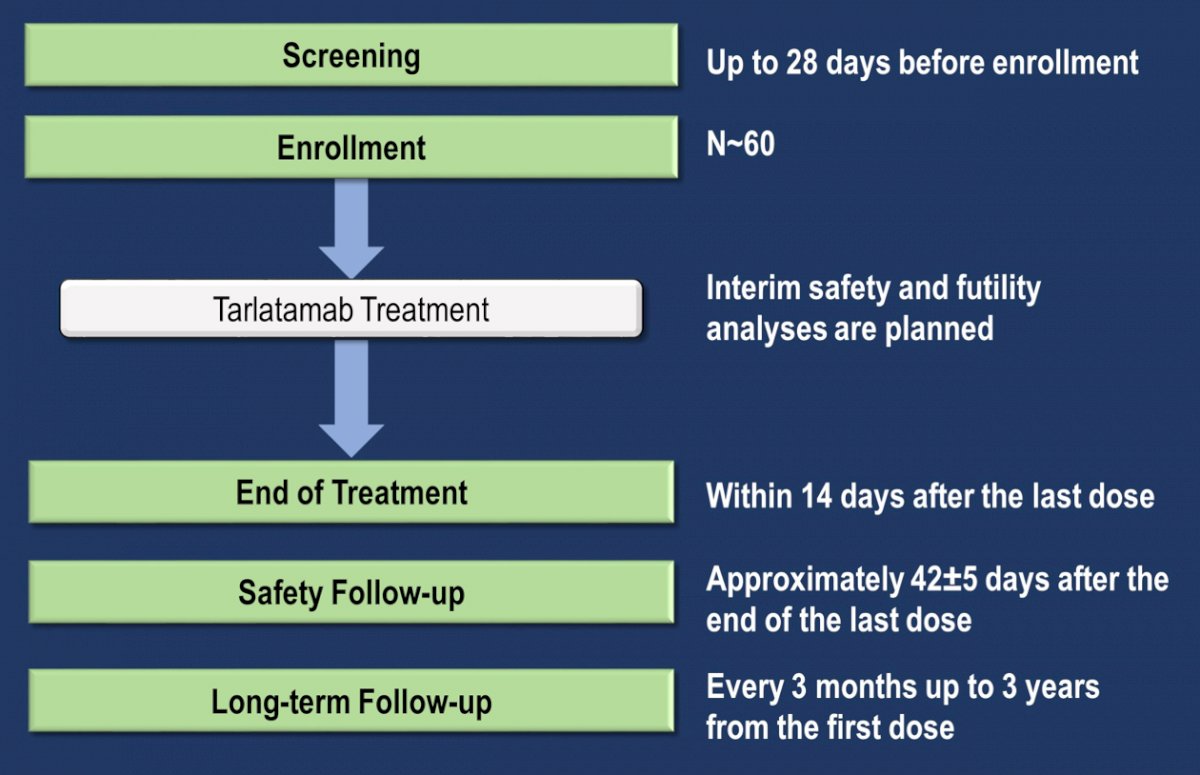
Key eligibility criteria include adults (≥18 y) with metastatic neuroendocrine prostate cancer whose disease progressed/recurred after ≥1 prior line of systemic therapy (platinum-based regimen for de novo neuroendocrine prostate cancer or an ASI if treatment-emergent), measurable disease per RECIST 1.1 with Prostate Cancer Working Group 3 modifications, and ECOG performance status ≤2. The primary objectives are to evaluate safety and tolerability and determine the maximum tolerated dose or recommended phase 2 dose of tarlatamab. Secondary objectives are to evaluate antitumor activity (as assessed by objective response, duration of response, progression-free survival, overall survival, and disease control rate) and characterize pharmacokinetics.
The first patient began treatment in June 2021, there are 8 patients enrolled and 16 sites activated in the US, Europe, Japan, and Australia as of January 2022.
Presented by: Rahul R. Aggarwal, UCSF Helen Diller Family Comprehensive Cancer Center, San Francisco, CA
Co-Authors: Sylvie Rottey, Ana Aparicio, Richard Greil, Melissa Andrea Reimers, Shahneen Kaur Sandhu, Yiran Zhang, Mark Salvati, Nooshin Hashemi Sadraei
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, Thursday Feb 17 – Saturday Feb 19, 2022


