(UroToday.com) The 2023 GU ASCO annual meeting included a session on urothelial carcinoma, featuring a presentation by Dr. Max Kates discussing updates from a phase 2 trial assessing intravesical gemcitabine and docetaxel in the treatment of BCG-naïve non–muscle invasive bladder cancer (NMIBC). Combination intravesical gemcitabine and docetaxel has demonstrated benefit for bacillus Calmette-Guérin (BCG) unresponsive NMIBC in retrospective series and is now being widely utilized as salvage therapy. BCG therapy is fraught with frequent drug shortages and some patients are unable to tolerate BCG due to side effects. Given ongoing BCG shortages as well as the promising efficacy and tolerability of gemcitabine and docetaxel as a salvage therapy, the objective of this study was to investigate the safety and efficacy of intravesical gemcitabine and docetaxel for high-risk BCG-naïve NMIBC in a prospective manner.
This study is an IRB-approved prospective single-arm open-label phase II trial for patients with BCG-naïve high-risk NMIBC. Intravesical gemcitabine (1,000 mg)/docetaxel (40 mg) in 100mL normal saline is given weekly for 6 weeks as induction followed by monthly maintenance therapy for 2 years among responders. The primary endpoint was 3-month complete response, defined as a negative bladder biopsy 6 weeks after induction treatment. Secondary endpoints included adverse events and 12-month complete response:

To date, the study has fully accrued with 25 patients enrolled from July 2020-August 2022. The pre-trial pathologic stages in this cohort were as follows: HGT1 with CIS (n = 7), HGT1 without CIS (n = 6), HGTa (n = 9), and CIS alone (n = 3). All 25 patients who completed induction therapy demonstrated complete response at 3 months. Thirteen out of 15 (87%) patients with at least 12 months of follow-up demonstrated continued complete response. Of those without a 12 month complete response, one patient had a HGTa recurrence at 9 months after coming off of maintenance treatment at month 4, and one patient has a high grade cytology without cystoscopic evidence of disease at month 9:
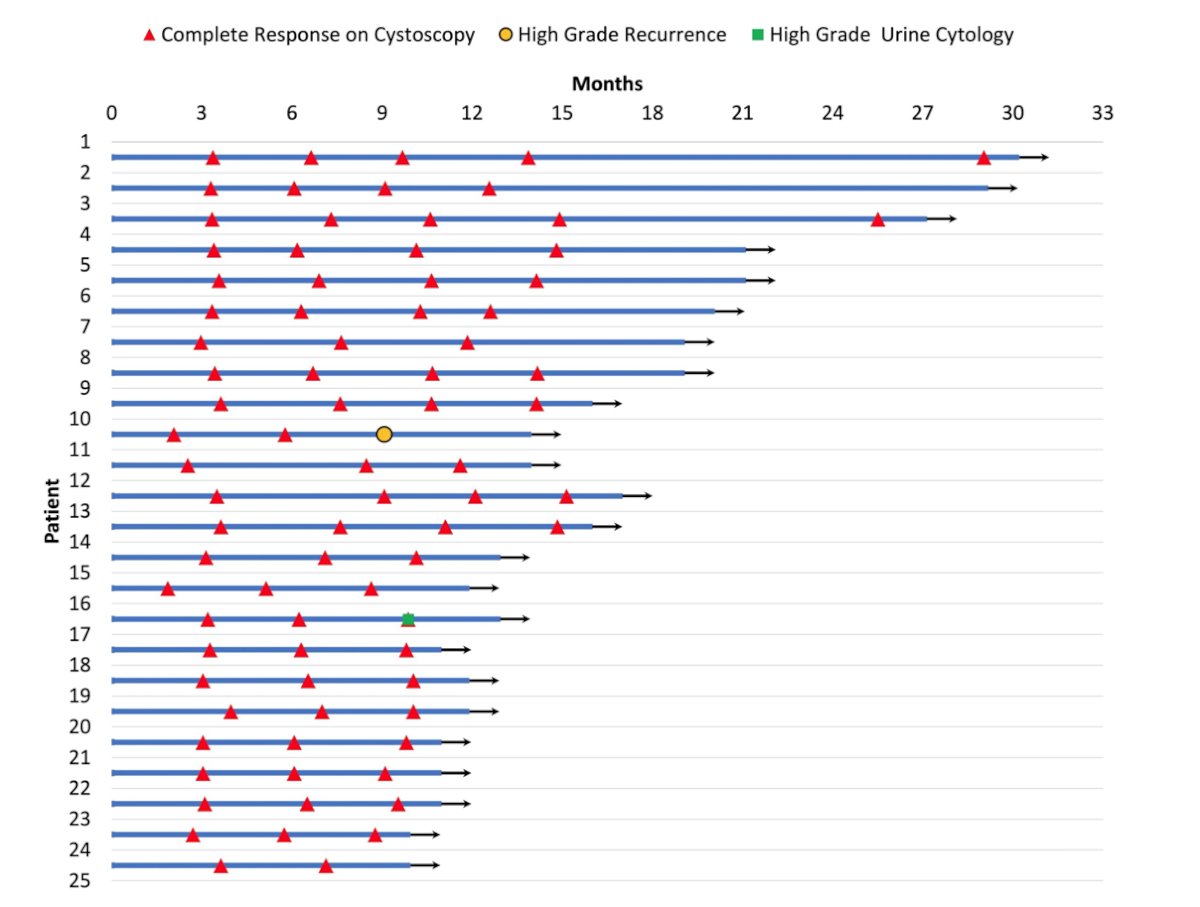
No enrolled patients have undergone radical cystectomy. Grade 1 adverse events were common (19/25 patients, 76%) including hematuria, urinary frequency, urgency, and fatigue. Two patients (8%) experienced a Grade 3 adverse events including urinary retention and UTI requiring hospitalization and intravenous antibiotics.
Dr. Kates concluded his presentation discussing updates from a phase 2 trial assessing intravesical gemcitabine and docetaxel in the treatment of BCG-naïve NMIBC with the following take-home messages:
- In this single-arm phase II trial, gemcitabine and docetaxel appears to be well-tolerated with promising short-term efficacy for patients with BCG-naïve high-risk NMIBC
- The EA8212 (BRIDGE) study is a phase 3 randomized controlled trial currently enrolling patients to either BCG or gemcitabine and docetaxel in high-risk NMIBC

Presented by: Max R. Kates, MD, James Buchanan Brady Urological Institute, Johns Hopkins University School of Medicine, Baltimore, MD
Co-Authors: Sunil H. Patel, Andrew Gabrielson, Connie Collins, Nirmish Singla, Trinity Bivalacqua, Noah M. Hahn
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2023 Genitourinary (GU) American Society of Clinical Oncology (ASCO) Annual Meeting, San Francisco, Thurs, Feb 16 – Sat, Feb 18, 2023.


