(UroToday.com) The 2023 American Society of Clinical Oncology Genitourinary (ASCO GU) cancers symposium held in San Francisco, CA between February 16th and 18th was host to a urothelial carcinoma rapid abstract session. Dr. Matt Galsky presented the co-primary endpoint analysis of HCRN GU 16-256, a phase II trial of gemcitabine, cisplatin, plus nivolumab with selective bladder sparing in patients with muscle-invasive bladder cancer.
Dr. Galsky began his presentation by recapping that cisplatin-based neoadjuvant chemotherapy (NAC) yields pathologic complete responses (pCR) in approximately 30 to 40% of patients with muscle invasive bladder cancer (MIBC).1 Furthermore, patients with pCR have significantly better survival outcomes.
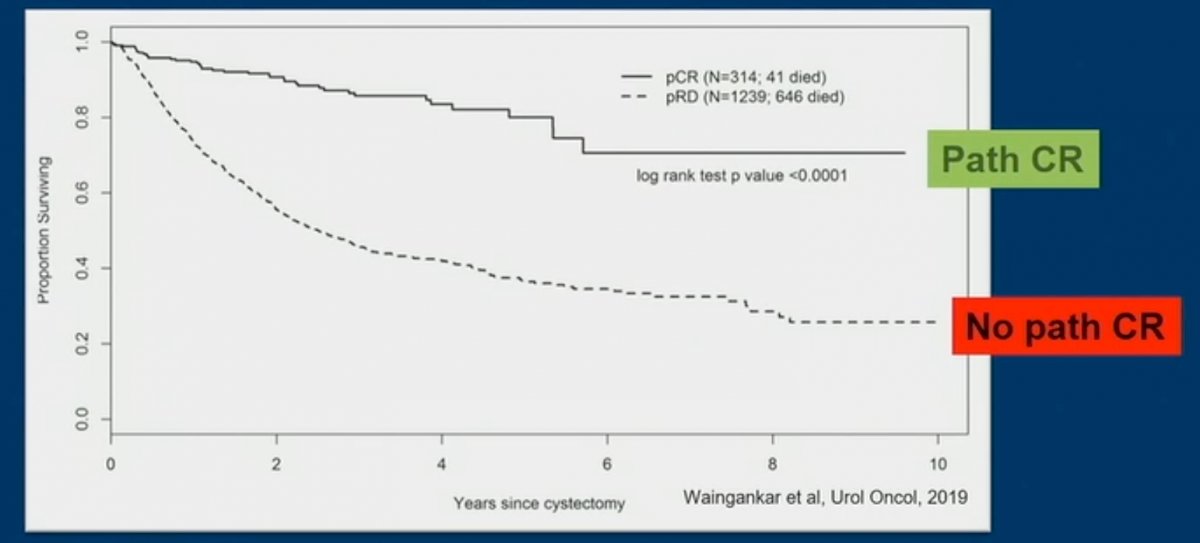
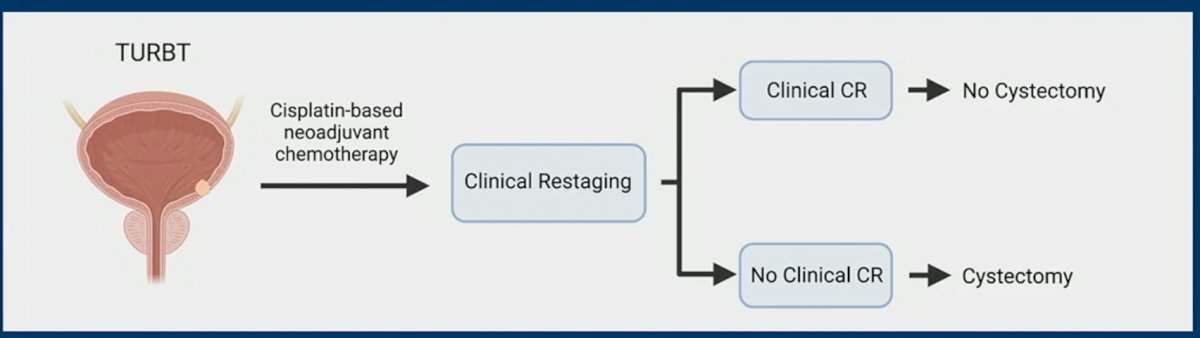
Unfortunately, as of date, a pCR can only be determined after the bladder has already been removed. One bladder-sparing paradigm that has been proposed it to treat MIBC patients with “neoadjuvant” chemotherapy, following which patients are clinically re-staged. Those with a clinical CR would avoid a radical cystetomy, whereas those without a CR would proceed to surgery.
Historical barriers to the adoption of this bladder-sparing paradigm include:
- Paucity of prospective studies
- Lack of rigorous methods to measure and define clinical CR
- Limited understanding of the role of “delayed” cystectomy in patients with local recurrence
- Suboptimal systemic therapeutic regimens
- Absence of biomarkers to refine decision making
As such, Dr. Galsky and colleagues designed the HCRN GU16-257 trial. As demonstrated in the figure below, this trial included patients with cT2-4aN0M0 cisplatin-eligible patients. All patients received the combination of gemcitabine + cisplatin + nivolumab for 4 cycles. Afterwards, patients were clinically restaged using cystoscopy, biopsies, urine cytology, and MRI. Patients with a clinical CR were offered either a radical cystectomy or no cystectomy (with a further 8 cycle of nivolumab akin to adjuvant ICI in patients post-cystectomy), per patient choice. Patients without a clinical CR proceeded to surgery.

The co-primary endpoints were:
- Clinical CR rate
- Performance of clinical CR in predicting treatment benefit, as defined by:
- 2-year metastasis-free if no cystectomy
- pCR in immediate cystectomy
This trial recruited 76 patients of whom 72 underwent clinical re-staging. Of these 72 re-staged patients, 33 (43%) had a clinical response and 32/33 chose against proceeding with cystectomy.

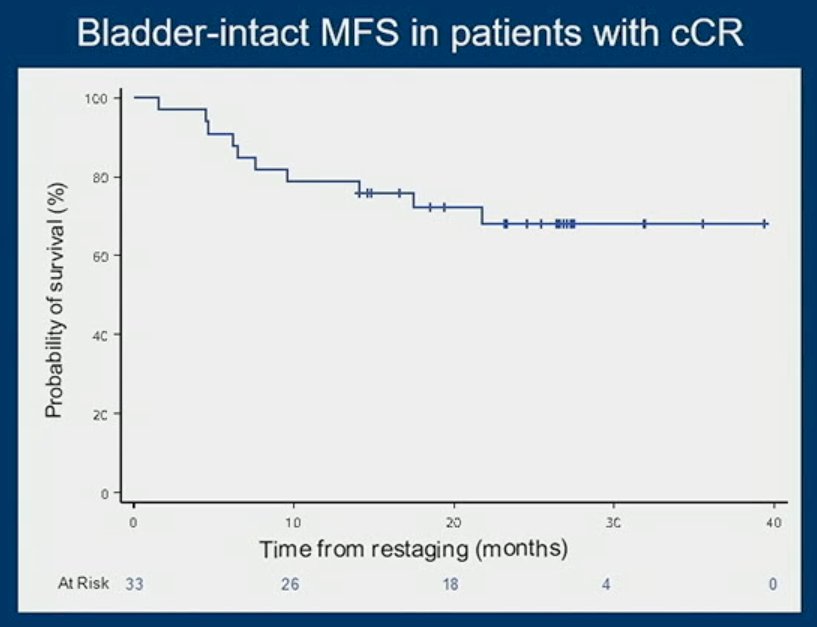
The plot below demonstrates the outcomes of patients achieving a clinical CR (n=33), with 9 patients eventually undergoing a delayed radical cystectomy, often after evidence of local recurrence, with 2/33 patients developing metastases. The median follow-up for these patients with a clinical CR was 30 months (range: 18 to 42 months). The bladder-intact MFS in this patient cohort is demonstrated below:

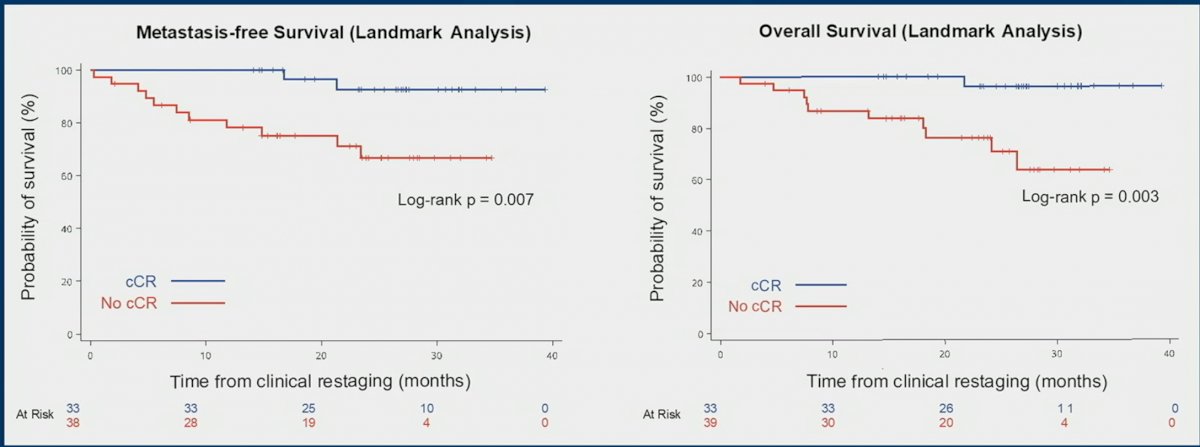
Patients with a clinical CR had significantly better MFS and OS as demonstrated in the KM curves below. Clinical CR predicted treatment benefit with appositive predictive value of 0.96 (95% CI: 0.89 – 1.0).
Analysis of TMB, including RB1, ATM, ERCC2, FANCC, and TP53, demonstrated that bladder-intact MFS in the overall cohort was significantly improved for patients with higher TMBs (>10):
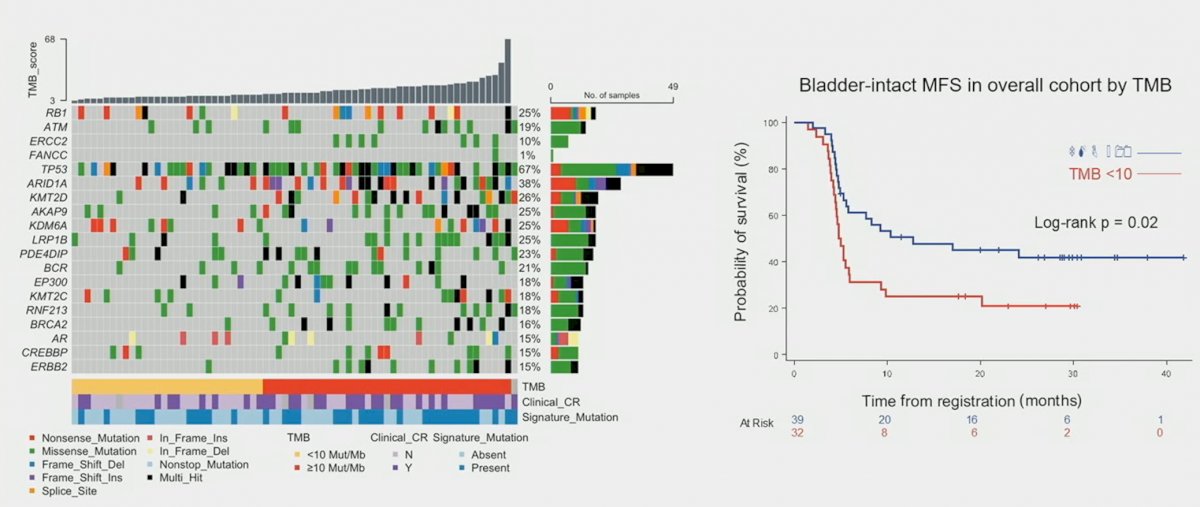
Dr. Galsky concluded his presentation as follows:
- Gemcitabine, cisplatin, plus nivolumab after TURBT was associated with a clinical CR rate of 43%
- Standardized clinical response assessment with a uniform complete CR definition identified patients with particularly favorable outcomes and facilitated bladder sparing
- These findings may help advance a more personalized approach to the management of MIBC leveraging clinical response-based risk stratification
Presented by: Matt D. Galsky, MD, FASCO, Professor of Medicine, Director of Genitourinary Medical Oncology, The Tisch Cancer Institute, Mount Sinai, New York, NY
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, San Francisco, CA, February 16th – February 18th, 2023
Reference:
Related Content:Gemcitabine, Cisplatin, and Nivolumab with Selective Bladder Sparing in MIBC: Co-Primary Endpoint Analysis of HCRN GU 16-256 Phase II Trial - Matthew Galsky


