(UroToday.com) The 2023 American Society of Clinical Oncology Genitourinary (ASCO GU) cancers symposium held in San Francisco, CA between February 16th and 18th was host to a urothelial carcinoma rapid abstract session. Dr. Jason Brown presented the results of HCRN GU14-188, a Phase Ib/II study of neoadjuvant pembrolizumab and chemotherapy for T2-4aN0M0 urothelial cancer.
Dr. Brown began by explaining that the study rationale was to improve upon the current standard of care for muscle-invasive urothelial carcinoma neoadjuvant treatment, particularly given the advances of immunotherapy effectiveness in urothelial cancer, and the synergy between immunotherapy and chemotherapy
The study design was as follows:
- Patients in cohort A (cisplatin eligible) received four 21-day cycles of gemcitabine and cisplatin, with pembrolizumab given on day 8 of these cycles, with an additional “5th dose of pembrolizumab” also subsequently given
- Patients in cohort B (cisplatin ineligible) received four 28-day cycles of gemcitabine and pembrolizumab, as demonstrated below.
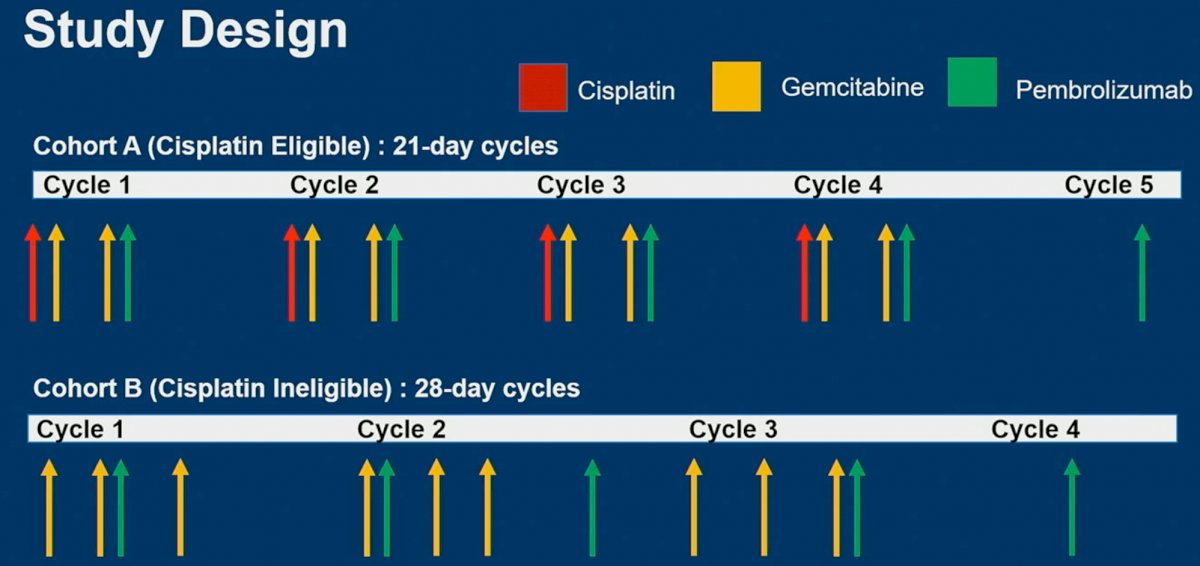
The primary endpoint was pathologic muscle invasive response (PaIR), with an a priori null hypothesis of a 23% PaIR in cohort A and 18% in cohort B. The secondary endpoints were:
- ypT0N0 rate
- 18-month recurrence-free survival
- 36-month overall survival
- Definitive surgery rate
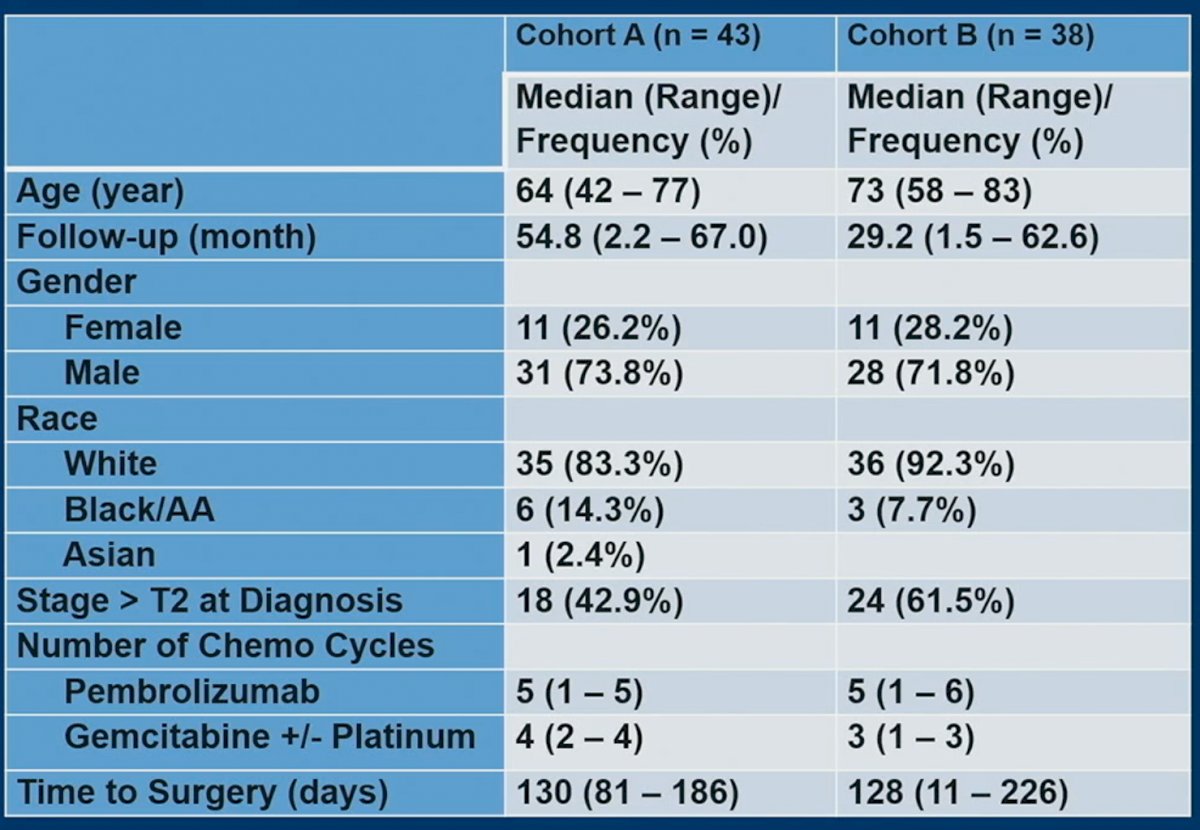
This trial enrolled 43 patients in cohort A and 38 in cohort B. Baseline patient characteristics are demonstrated below. Of note, stage >T2 at diagnosis was present in 43% of patients in cohort A and 61.5% in cohort B. The number of pembrolizumab cycles received in both cohorts was 5, and the number of gemcitabine +/- cisplatin was, as designed, 4 and 3, respectively. The median time to surgery was 128 – 130 days.
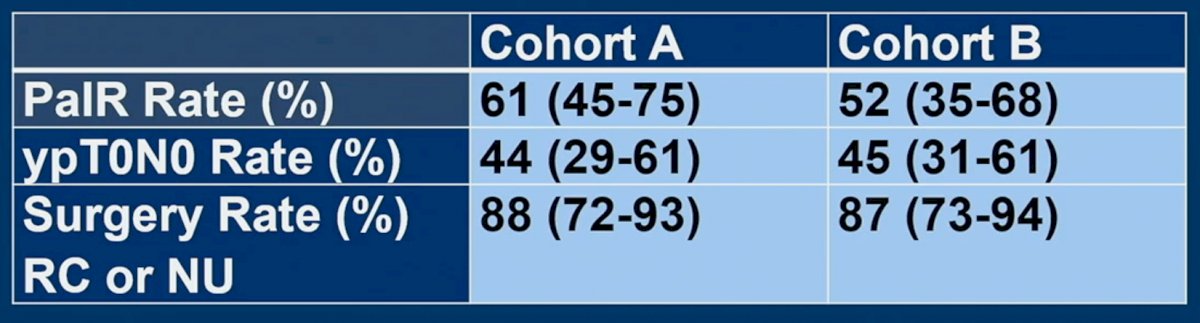
The primary endpoint of PaIR rate was observed in 61% of patients in cohort A and 52% of patients in cohort B. The ypT0N0 proportions were 44% and 45%, respectively. 88 and 87% of patients in cohorts A and B, respectively, underwent subsequent surgery in the form of a radical cystectomy or nephroureterectomy.
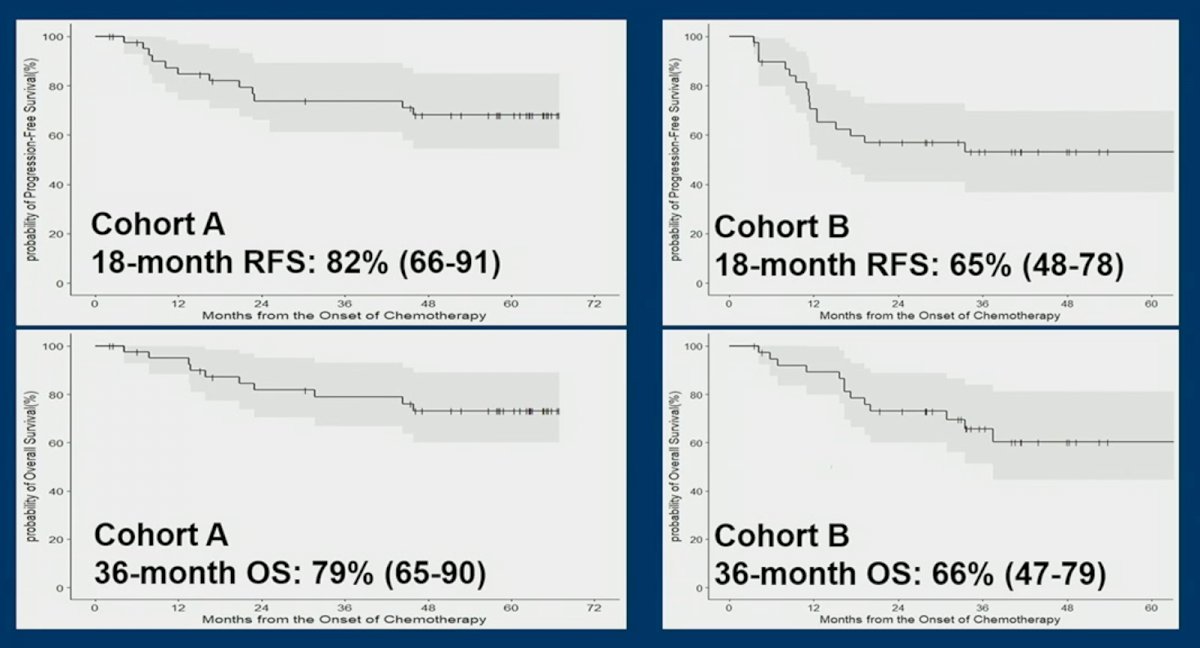
18-month RFS in the cohorts were as follows:
- Cohort A: 82% (95% CI: 66- 91%)
- Cohort B: 65% (95% CI: 48 – 78%)
36- month OS in the cohorts were as follows:
- Cohort A: 79% (95% CI: 65 – 90%)
- Cohort B: 66% (95% CI: 47 – 79%)
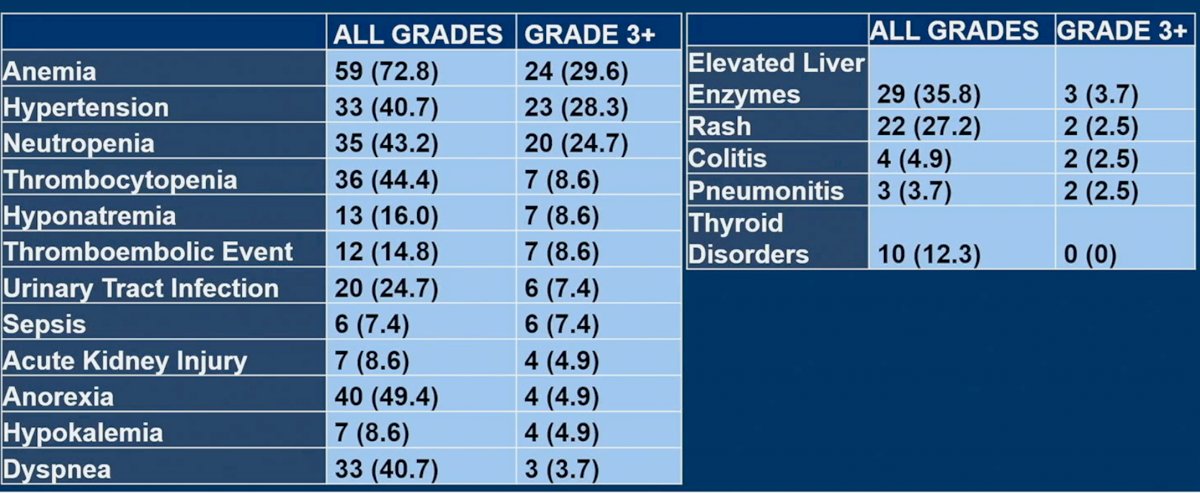
With regards to toxicities, the most frequent grade 3+ AEs were:
- Anemia: 29.6%
- Hypertension: 28.3%
- Neutropenia: 24.7%
Dr. Brown concluded as follows:
- This trial met its primary endpoints in both cohorts
- Excellent survivals with sustainable responses were observed
- The regimens were well-tolerated
- Ongoing studies will further evaluate efficacy of chemoimmunotherapy in muscle-invasive urothelial carcinoma
Presented by: Jason Brown, MD, PhD, Assistant Professor, Division of Oncology, University Hospitals Seidman Cancer Center, Cleveland, OH
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, San Francisco, CA, February 16th – February 18th, 2023


