(UroToday.com) In this trial, Gaffney et al. report on a Phase II trial of intravesical chemoimmunotherapy (gemcitabine and bacillus Calmette-Guérin (BCG)) for patients with BCG-exposed, high-grade, non-muscle–invasive bladder cancer.
As background, the authors note that the standard of care (SOC) for high-risk non-muscle invasive bladder cancer (NMIBC) after exposure to induction only BCG or relapse >12 months after “adequate” BCG is retreatment with BCG. However, ~50% of patients will relapse within 6 months. Thus, there is a critical need to develop novel combination therapies to improve BCG immunotherapy efficacy.
Combination of BCG with immune checkpoint inhibition is associated with a ~12-18% risk of serious treatment-related adverse events while BCG combined with Mitomycin C (MMC) has unacceptable urinary toxicity.
Intravesical Gemcitabine (GEM) is a common treatment for NMIBC, is well tolerated, has better efficacy after BCG than MMC, and, based on preclinical data, may synergistically enhance the efficacy of BCG by augmenting the immune tumor microenvironment.
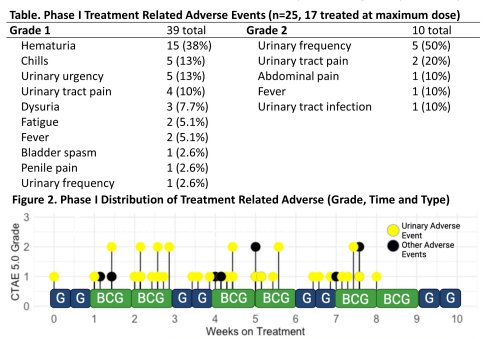
The authors had previously completed a prospective Phase I trial of chemoimmunotherapy with gemcitabine and BCG in BCG-relapsed/BCG-exposed NMIBC that was well-tolerated (no treatment related grade ≥3 adverse events) with promising early efficacy (95% [19/20] 6-month complete response rate). No dose limiting toxicities were seen. The maximum tolerated dose (MTD) was 2000 mg gemcitabine and 50 mg TICE BCG.
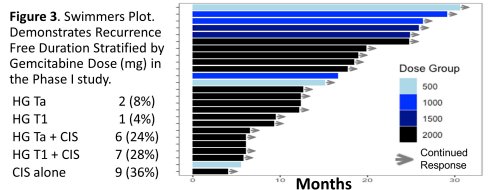
In the Swimmer’s plot below, DF duration stratified by Gem dose in the Phase 1 study:
Based on this phase 1 trial, this phase 2 investigator initiated trial was started.
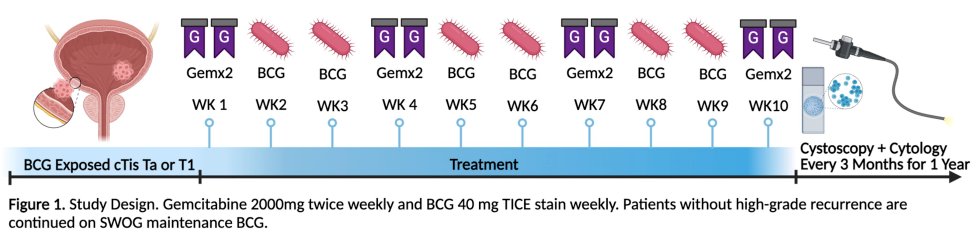
The figure below highlights the study design:
The Phase II portion is currently enrolling patients with
- BCG-exposed carcinoma in-situ (CIS ±Ta/T1) that recurred within 24 months of last BCG (BCG-exposed/BCG-relapsing NMIBC).
- Patients with BCG-unresponsive disease, contraindication to BCG, or prostatic/ureteral urothelial disease are excluded
Induction intravesical GEM (2000mg, twice weekly) is given weeks 1, 4, 7, and 10 and intravesical BCG (40mg, Tice strain, weekly) is given weeks 2, 3, 5, 6, 8, and 9. Responders receive SWOG maintenance BCG.
Primary endpoint is clinical complete response (CR) at 6 months (absence of high-grade disease on cystoscopy/TURBT and negative cytology) using a Simon’s optimal 2-stage design testing the null hypothesis of a true CR of 55% at 6 months (based on historical outcomes of BCG alone) against a 1-sided alternative.
- In the first stage, the study would stop if ≤ 9/15 patients had a CR.
- In the second stage, the null hypothesis will be rejected if ≥ 29 CRs are observed in 43 total patients (type I error rate 5% and power of 80% if the true CR rate is 75%).
- Phase I patients treated at the MTD (14/25) are included in Phase II.
Secondary outcomes include recurrence-free, progression-free, and cystectomy-free survival.
Correlative studies explore immune and molecular predictors of response and resistance to chemoimmunotherapy in tumor tissue, urine, and blood
The expected accrual is 43 patients.
Presented by: Christopher Gaffney, MD, Memorial Sloan Kettering Cancer Center, New York, NY
Written by: Thenappan (Thenu) Chandrasekar, MD – Urologic Oncologist, Associate Professor of Urology, University of California, Davis @tchandra_uromd on Twitter during the 2023 Genitourinary (GU) American Society of Clinical Oncology (ASCO) Annual Meeting, San Francisco, Thurs, Feb 16 – Sat, Feb 18, 2023.


