(UroToday.com) The immune checkpoint inhibitor pembrolizumab is the current standard of care for patients with metastatic urothelial carcinoma (UC) who progress following first-line platinum-based chemotherapy, yet only approximately 21% of patients will respond.1 These background data demonstrate the unmet clinical need for treatment of patients with metastatic UC in the post-platinum setting.
Among currently options is sacituzumab govitecan (SG), an antibody-drug conjugate which combines a humanized monoclonal antibody (sacituzumab, hRS7 IgG1k) directed against Trop-2 covalently linked to a topoisomerase I inhibitor (SN-38). Its target, Trop-2, plays significant roles in cell migration and anoikis. SG has demonstrated previously significant activity (27% ORR) and manageable safety in Cohort 1 (n=113) of TROPHY-U-01,2 which led to accelerated FDA approval. Cohort 3 of the study is a phase II evaluation of second-line treatment with SG plus pembrolizumab in platinum-exposed checkpoint inhibitor-naïve patients with metastatic UC. The authors have previously reported preliminary efficacy of TROPHY-U-01 cohort 3 at ASCO 2022,3 and here present the primary analysis (schema of TROPHY-U below, NCT03547973).
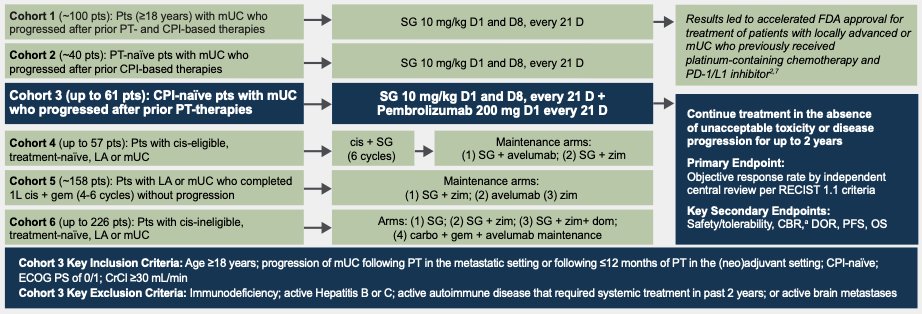
Dr. Grivas and colleagues reported updated results of the single arm TROPHY-U-01 Cohort 3. Eligibility for this cohort included prior progression of metastatic UC following platinum-based chemotherapy (in the metastatic setting) or ≤12 months following neoadjuvant/adjuvant platinum in the curative intent. No prior checkpoint inhibitor exposure was allowed. All patients had an ECOG performance status of 0-1. Treatment regimen was a 10 mg/kg of SG on D1 and D8 and 200 mg of pembrolizumab on D1 of a 21-day cycle for up to two years. The primary endpoint was objective response rate (complete response [CR] + partial response [PR]) per central review by RECIST 1.1. Secondary endpoints include clinical benefit rate (CBR; CR + PR + stable disease for at least 6 months), duration of response (DOR), and progression-free survival (PFS) per central review, as well as safety. Target enrollment was approximately 41 patients based on a Simon two-stage design for 90% power at one-sided alpha of (0.05) to demonstrate a 21% improvement in ORR, based on a historical ORR of ≤20% and alternative hypothesis of ≥41% ORR. In keeping with a two-stage design if ≥13 responses are observed among all 41 patients, the null hypothesis will be rejected. If ≥17 responses are observed, then the target ORR will be met. The Kaplan-Meier method was used to analyze time-to-event endpoints and descriptive statistics were used to characterize and present adverse events (AEs) in safety analyses.
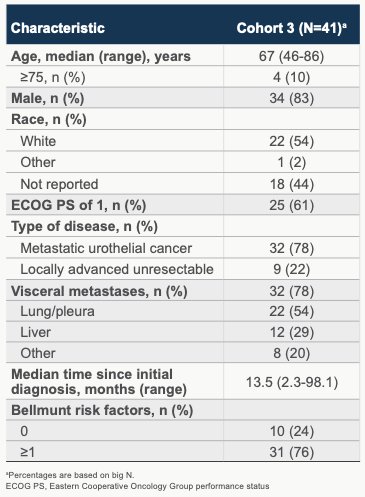
A total of 55 patients were enrolled and 41 were treated. Data cutoff for this presentation of results was July 26, 2022, which allowed for a median follow-up was 12.5 months (range, 0.9-24.6) for all treated patients. Baseline characteristics were summarized in table form as shown. All patients had prior platinum-based chemotherapy with a median of 1 prior anticancer regimen received. 17 of 41 (41.5%) of patients received last prior therapy in the neoadjuvant therapy setting. Most patients (71%, 29/41) received cisplatin with the balance receiving carboplatin. The median duration of last prior anti-cancer therapy was 2.7 months (range, 0-13), although this number was impacted by those patients treated previously in the metastatic setting (24 patients, median time since last systemic therapy to screening date of 2.0 months (range 0.3-60.6 months). Best response to prior systemic platinum therapy in the metastatic intent group was as follows: CR (1, 4%), PR (2, 8%), SD (13, 54%), PD (6, 25%), with 2 (8%) not reported/not available.
Per central independent review, the primary endpoint was met with a 41% ORR (20% CR rate and 46% clinical benefit rate), with breakdown as shown:
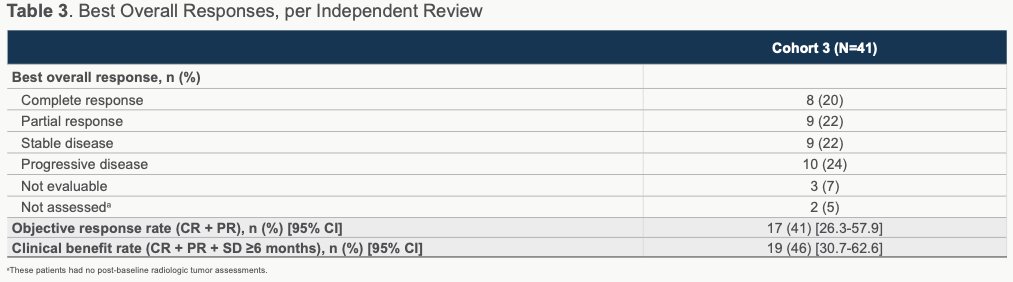
The authors note that ORRs were largely similar across prespecified subgroups, which included those patients with visceral and liver metastases and Bellmunt risk groups. Among those patients evaluable, 82% (28/39) experience reduction in target lesion size.
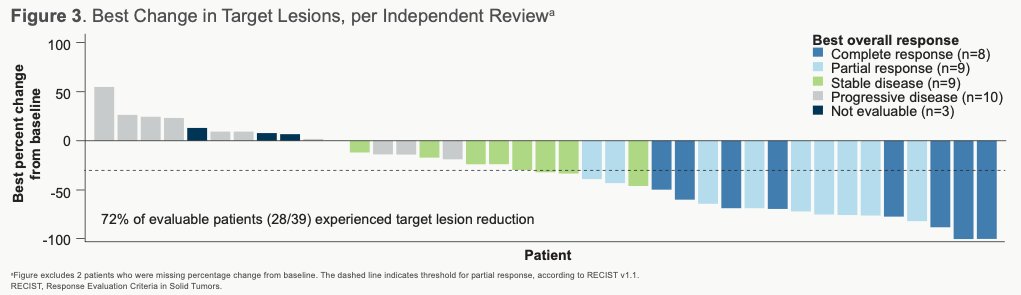
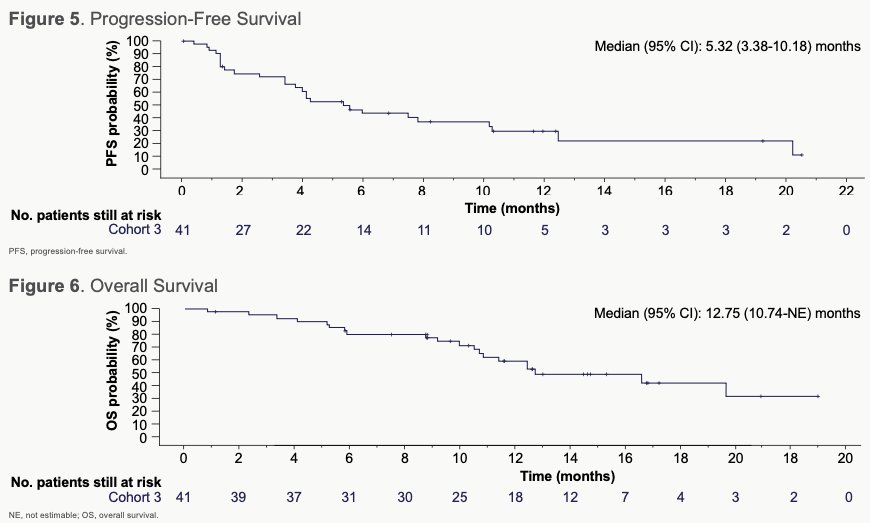
For the majority of responders (and some non-responders), the best reduction in size of target lesions from baseline was observed to be durable. The median duration of response was 11.1 months (95% CI, 4.8-NE [not estimable]; n=17) and median PFS was 5.3 months (95% CI, 3.4-10.2). Median time to response was 1.4 months (95% CI, 1.3-2.7) and median OS was 12.7 months (95% CI, 10.7-NE).
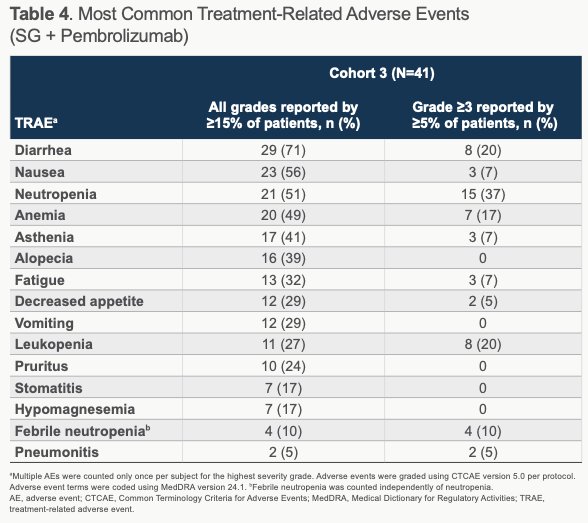
Safety assessments demonstrated that all treated patients experienced at least 1 treatment-related AE (TRAE) and 61% experiencing a grade ≥3 TRAE, of which neutropenia (37%; 10% febrile neutropenia), leukopenia (20%), and diarrhea (20%) were the most common. TRAEs prompted reductions in SG dose in 39% of patients and discontinuation in 15%. The most commonly observed any grade AEs related to pembrolizumab were diarrhea (24%), asthenia (22%), pruritis (20%), and fatigue (17%). Five patients received systemic steroids for pembrolizumab-related AEs. Granulocyte colony-simulating factor (G-CSF) use was common, with 9 (22%) using it for prevention of AE and 8 (20%) using it for treatment of an AE. No treatment-related deaths occurred.
In conclusion, the authors demonstrate that the combination of SG and pembrolizumab resulted in high ORR and clinical benefit rate, as well as a manageable safety profile, as second line treatment of patients with metastatic UC following platinum-based chemotherapy exposure and without prior immune checkpoint inhibitor treatment. Combination of these two drugs, with distinct mechanisms of action, did not result in any new safety signals. Ongoing pursuit of this combination for metastatic UC is anticipated.
Presented by: Petros Grivas, MD, Ph.D, University of Washington, Fred Hutchinson Cancer Center, Seattle, WA
Written by: Jones Nauseef, MD, PhD, Assistant Professor of Medicine within the Division of Hematology and Medical Oncology, Sandra and Edward Meyer Cancer Center, and Englander Institute for Precision Medicine Weill Cornell Medicine and Assistant Attending physician at NewYork-Presbyterian Hospital. @DrJonesNauseef on Twitter during the 2023 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, Thursday Feb 16 – Saturday Feb 18, 20223
References:
- Bellmunt J, et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N Engl J Med. 2017;376(11):1015-1026.
- Tagawa ST, et al. TROPHY-U-01: A Phase II Open-Label Study of Sacituzumab Govitecan in Patients With Metastatic Urothelial Carcinoma Progressing After Platinum-Based Chemotherapy and Checkpoint Inhibitors. J Clin Oncol 2021;39:2474-2485.
- Grivas P, et al. TROPHY-U-01 Cohort 3: Sacituzumab govitecan (SG) in combination with pembrolizumab (Pembro) in patients (pts) with metastatic urothelial cancer (mUC) who progressed after platinum (PLT)-based regimens. J Clin Oncol. 2022;40(6 suppl):434-434.
TROPHY-U-01 Cohort 3 Primary Analysis Sacituzumab Govitecan in Combination with Pembrolizumab in Patients with Metastatic Urothelial Cancer That Progressed after Platinum-Based Therapy - Petros Grivas


