(UroToday.com) The 2023 GU ASCO annual meeting included a session on addressing challenges to ensure health equity in bladder cancer, featuring a presentation by Dr. Elad Sharon discussing health equity by expanding trial eligibility criteria in urothelial carcinoma. Dr. Sharon started by highlighting the NCI’s 2030 vision for clinical trials, specifically the CTAC strategic planning working group report. This report focused on recommendations selected for initial implementation, including (i) streamlining clinical trials, (ii) decentralized trial activities, and patient access to clinical trials, such as broadening eligibility criteria, and conducting trials that support minority and unserved patient needs. The rationale for broadening eligibility criteria is that higher rates of chronic comorbidities are seen in minority and underserved populations, which limit their participation in clinical trials. The recommendation from the group is to broaden eligibility criteria to address distinctive medical problems experienced by minority and underserved patients.
Eligibility criteria are necessary in clinical trials to define the patient population under study, isolating the potential effect of an investigational drug, and ensuring that the trial is conducted safely. However, excessive or overly rigid eligibility criteria may impair the rate of trial accrual, restrict patient access to investigational drugs, and limit the ability to generalize the results to the broader population of patients who will ultimately use the drug.
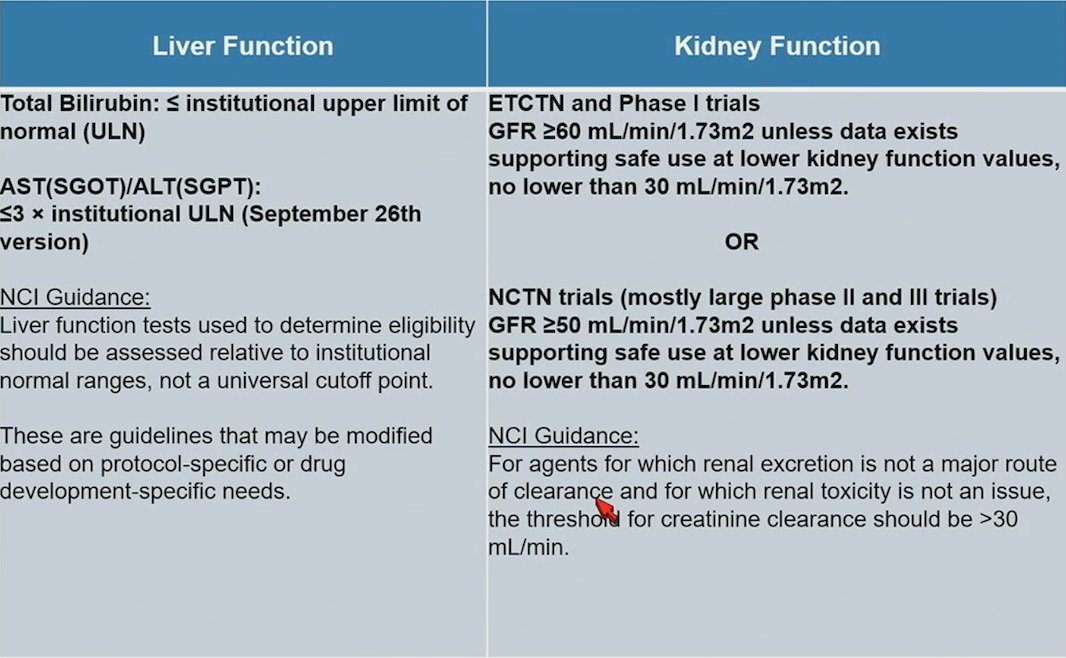
In 2016, ASCO and Friends of Cancer Research began a joint project to develop and advance specific strategies to change the exclusionary nature of eligibility trials. This included for patients with brain metastases, HIV/AIDS, organ dysfunction/prior and concurrent malignancies, and minimum age for enrollment. Subsequently, an ASCO-Friends joint research statement and four supporting manuscripts on the topic were published in the Journal of Clinical Oncology. The National Cancer Institute’s Cancer Therapy Evaluation Program added to its Generic Protocol Template in September 2018, and February 2022 followed the ASCO-Friends recommendations closely. In July 2020, the US Food and Drug Administration published four final Guidance for Industry documents related to cancer clinical trial eligibility criteria. The NCI Inclusion Criteria Template Language and Guidance based on the joint ASCO/Friends Recommendations for liver and kidney function is as follows [1]:
In the ASCO-Friends May 2021 eligibility criteria update, key recommendations were made for laboratory reference ranges and test intervals:

Specifically, with regards to laboratory references ranges:
- Laboratory test results should only be used as exclusion criteria when scientifically justified and when abnormal test results confer safety concerns
- Laboratory reference values should account for potential normal variations due to race, ethnicity, age, sex, and gender identity (ie. due to surgical and/or hormonal changes)
- Routine assessment of laboratory test-based exclusion criteria should be conducted during the course of clinical research and drug development as investigational agents progress from earlier to later-phase clinical trials
Next, Dr. Sharon discussed why we should care about GFR, which includes trial and drug eligibility, drug dosing (ie. continuous for carboplatin, dose reductions for other drugs), and drug discontinuation. We are able to measure GFR by collecting 24 hour urine or using the following formulas to inform production rate (muscle): Cockcroft-Gault, MDRD, and CKD-EPI. With regards to Cockcroft-Gault estimates, the formula is based on data from adult males, and because of different relative amounts of fat and muscle in women, correction is required, which has been estimated at a 15% reduction. Additionally, previous recommendations were to multiply by 1.159 for Black/African American patients, however, some institutions have removed this requirement. By inappropriately calculating Black/African American eGFR, this may reduce the proportion of Black patients eligible to receive anticancer drugs, and up to 18% would have received discordant drug dosing or eligibility recommendations.
Finally, there has been a push for ancestry-driven recalibration of tumor mutational burden with respect to immune checkpoint inhibitors. Across tumor-only panels, tumor mutational burden inflation is more pronounced in non-Europeans, and modification of tumor-only tumor mutational burden using paired tumor/normal tumor mutational burden improves ancestral biases. Choice of tumor mutational burden can affect outcomes for enrolled populations of non-European patients, and false representation of tumor mutational burden status adversely affects outcomes and worsen disparities.
Dr. Sharon concluded his presentation by discussing health equity by expanding trial eligibility criteria in urothelial carcinoma with the following concluding messages:
- Expanding eligibility criteria on clinical trials is an essential means to improving health equity: populations on trial are noted on labels in public reports, and exclusions lead to treatment dilemmas and uncertainty
- Changes in standard creatinine clearance calculators can have unintended deleterious effects on trial eligibility of underserved populations
- Laboratory test results should only be used as exclusion criteria when scientifically justified and when abnormal test results confer safety concerns. When those safety concerns are addressed or managed, then the criteria should be broadened. Continuing strict criteria in the absence of adequate justification diminishes health equity
- Vigilance is required to minimize bias when it is discovered and finding solutions is critical (ie. germline/tumor paired sequencing for ancestry-informed tumor mutational burden)
Presented by: Elad Sharon, MD, PhD, National Cancer Institute, Bethesda, MD
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2023 Genitourinary (GU) American Society of Clinical Oncology (ASCO) Annual Meeting, San Francisco, Thurs, Feb 16 – Sat, Feb 18, 2023.
References:
- Kim ES, Uldrick TS, Schenkel C, et al. Continuing to Broaden Eligibility Criteria to Make Clinical Trials More Representative and Inclusive: ASCO-Friends of Cancer Research Joint Research Statement. Clin Cancer Res. 2021 May 1;27(9):2394-2399.


