(UroToday.com) The 2023 American Society of Clinical Oncology Genitourinary (ASCO GU) cancers symposium held in San Francisco, CA between February 16th and 18th was host to a urothelial carcinoma rapid abstract session. Dr. Daniel Petrylak presented the results of the TROPHY-U-01 Cohort 2, a phase II study of Sacituzumab Govitecan in platinum-ineligible patients with metastatic urothelial carcinoma who had progressed after prior checkpoint inhibitor therapy.
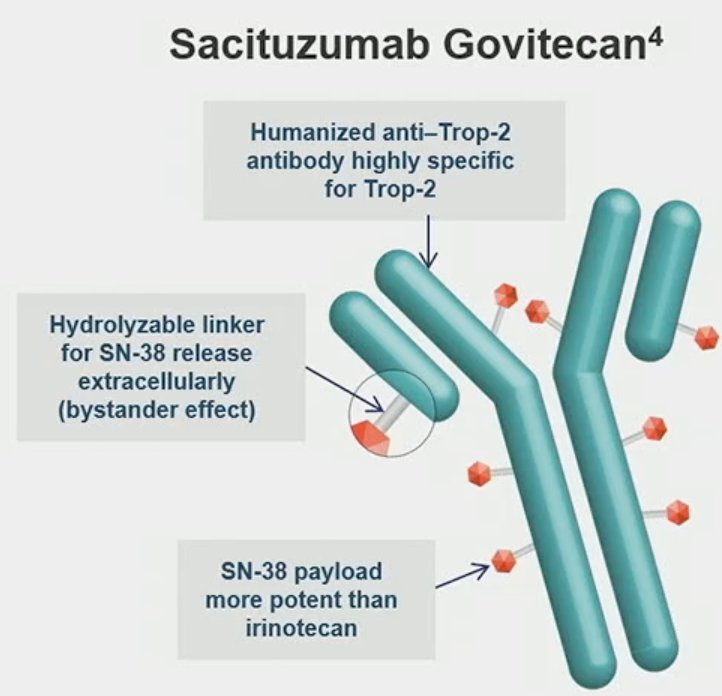
Dr. Petrylak began by highlighting that patients with locally advanced or metastatic urothelial carcinoma who are ineligible for platinum-based chemotherapy and have progression after checkpoint inhibitor therapy have limited therapeutic options and an overall poor prognosis.1 Sacituzumab Govitecan (SG) is a novel Trop-2-directed antibody-drug conjugate (ADC) that demonstrated an objective response rate of 27%, a median duration of response of 7.2 months, a median OS of 10.9 months, and a manageable safety profile in 113 patients with locally advanced or metastatic urothelial carcinoma who had progression after platinum and checkpoint inhibitor therapies in the pivotal TROPHY-U-01 Cohort 1 study, leading to accelerated FDA approval in this patient population.2,3 In this this analysis, Dr. Petrylak reported the primary analysis of TROPHY-U-01 Cohort 2, a phase 2 study of SC in patients with metastatic urothelial carcinoma who progressed after checkpoint inhibitors but were platinum-ineligible at the start of the study.
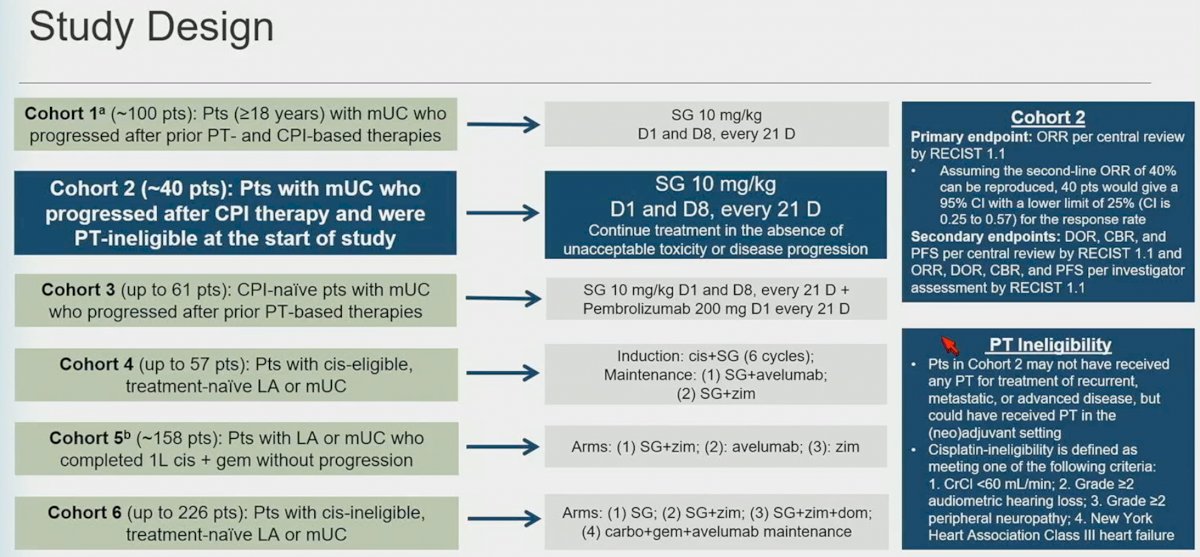
There are currently 6 cohorts as part of the TROPHY-U-01 umbrella design. As highlighted below, Cohort 2 was planned to include ~40 patients, with a treatment regimen of SG 10 mg/kg, on days 1 and 8, every 21 days. Treatment would be continued until unacceptable toxicity or disease progression occurred. The primary endpoint was ORR per central review by RECIST 1.1. Assuming the second-line ORR of 40% can be reproduced, 40 patients would give a 95% CI with a lower limit of 25% (CI: 0.25 – 0.57) for the response rate. Secondary endpoints included:
- Duration of response (both by central review and investigator assessment using RECIST 1.1 criteria for both)
- Clinical benefit rate(both by central review and investigator assessment using RECIST 1.1 criteria for both)
- Progression-free survival (both by central review and investigator assessment using RECIST 1.1 criteria for both)
- ORR (investigator assessment using RECIST 1.1 criteria)
Exclusion criteria included:
- Receipt of any platinum chemotherapy for treatment of recurrent, metastatic, or advanced disease. They could have received in the neoadjuvant setting.
Cisplatin ineligibility was defined using the Galsky criteria.
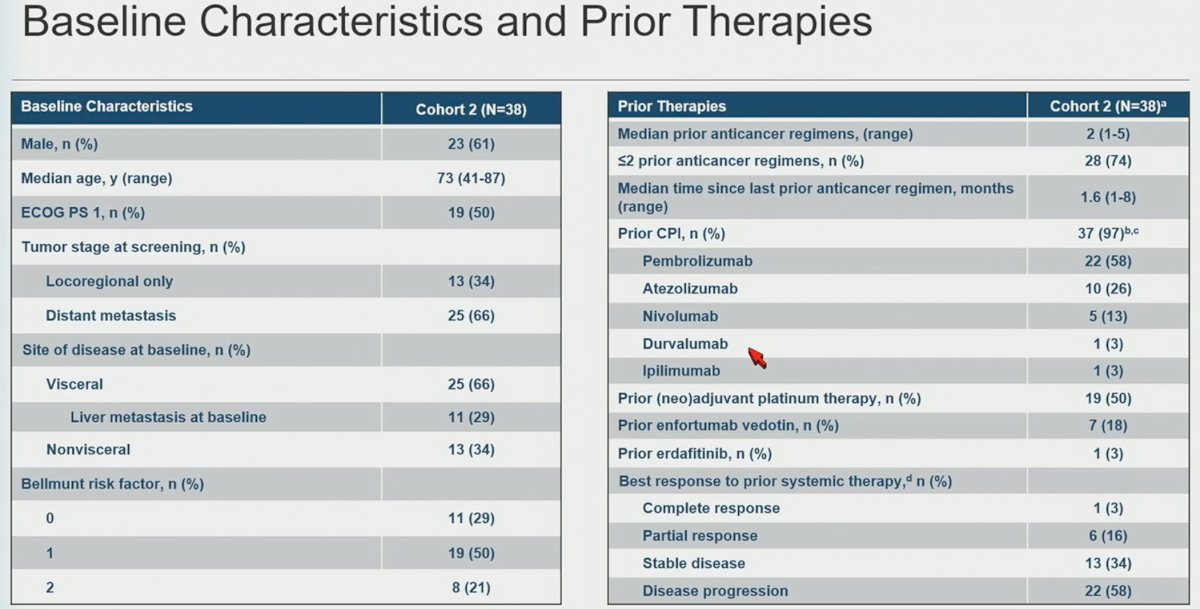
38 patients were included in this cohort. Median age was 73 years, 66% had visceral metastases, the median number of prior anticancer regimens was 2 (range: 1 – 5), the median time since last prior regimen was 1.6 months (range: 1 – 8 months), pembrolizumab (58%) was the most common prior checkpoint inhibitor used, 50% had received prior neoadjuvant chemo, 18% prior enfortumab vedotin, and 3% erdafitinib. 19% had a prior complete or partial response as a best response to prior systemic therapy.
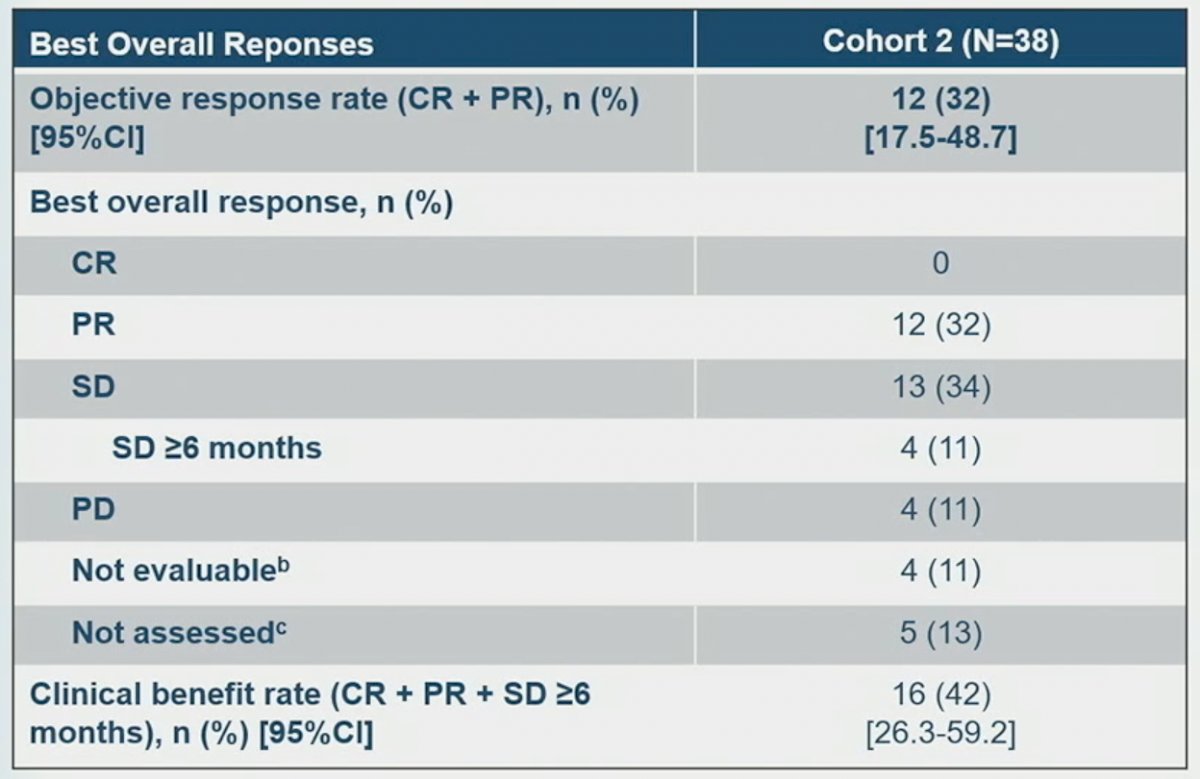
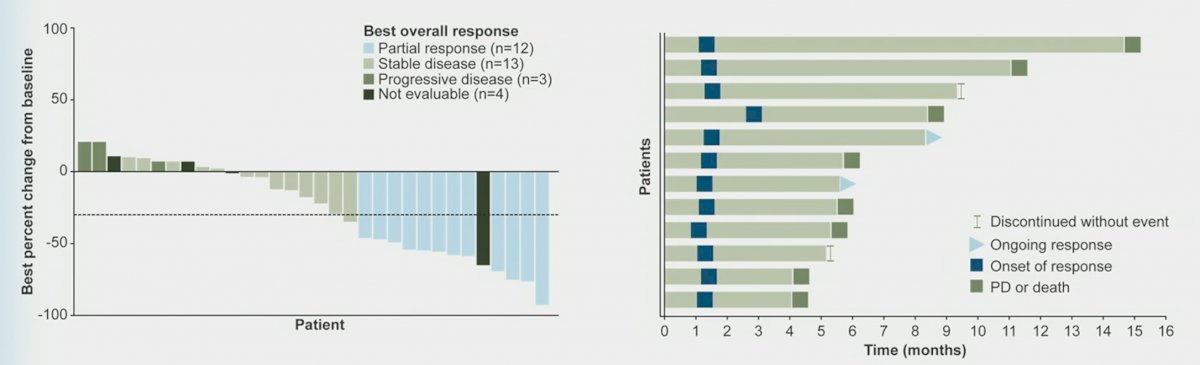
An objective response, defined as complete or partial response, was observed in 12 patients (32%). No patient had a complete response. Stable disease was observed in an additional 13 patients (34%), with 4 (11%) having stable disease for at least 6 months. The overall clinical benefit, defined as CR, PR, or SD for at least 6 months, was observed in 16 patients (42%) The median time to response was 1.4 months and the median duration of response was 5.6 months (95% CI: 2.8 – 13.3 months). ORRs were similar across prespecified subgroups, regardless of number of prior anticancer therapies, though some subgroups had limited patient numbers. Of note, an ORR of 53.8% was observed in patients without prior platinum or enfortumab vedotin exposure (n=13).
69% of assessed patients (22/32) experienced target lesion reduction. Two patients had an ongoing response at data cutoff.
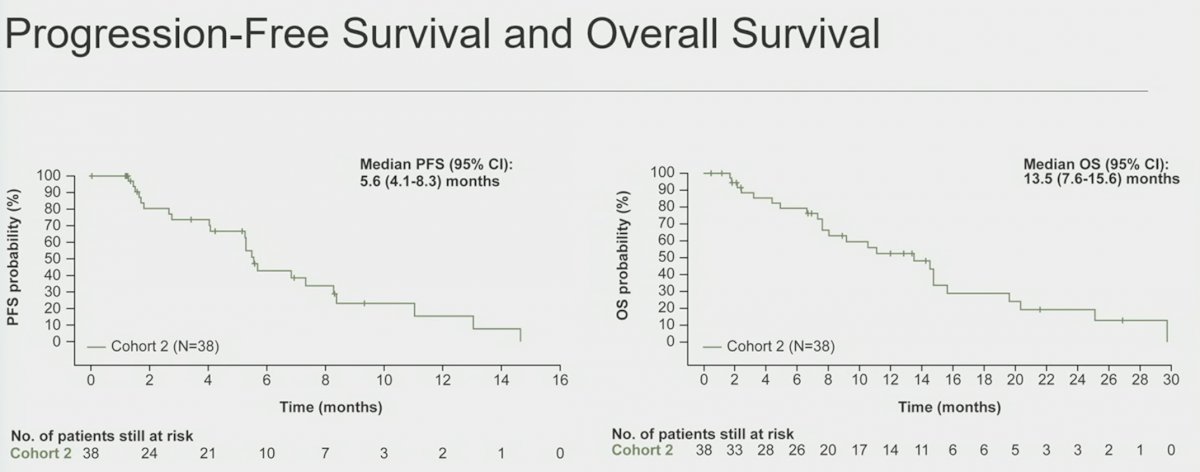
At a median follow-up of 9.3 months, the median PFS was 5.6 months (95% CI: 4.1 – 8.3) and median OS was 13.5 months (95% CI: 7.6 – 15.6).
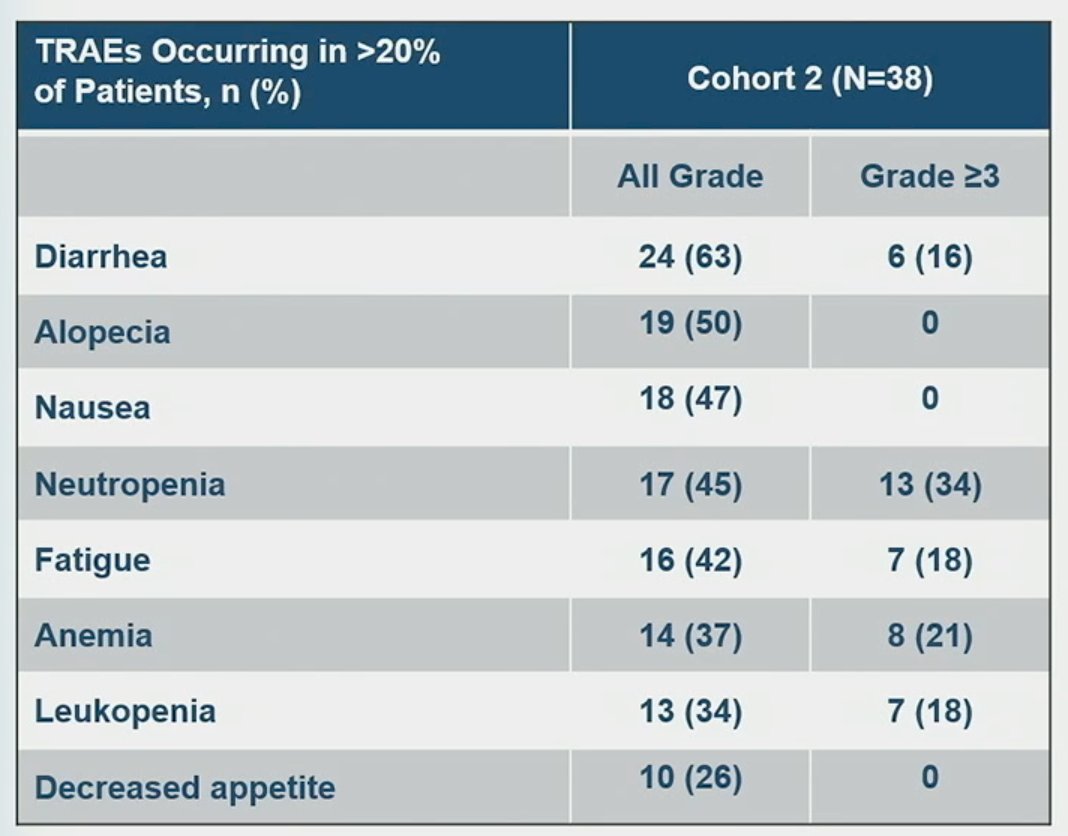
With regards to safety outcomes, 26 (68%) patients had grade 3 or worse treatment-related AEs. The most common of which were neutropenia (34%), anemia (21%), leukopenia (18%), fatigue (18%), and diarrhea (16%). Three (8%) patients had treatment-related febrile neutropenia (2 with grade 3 and 1 with grade 4). 14 patients (37%) had SG dose reduction due to TRAEs, and 7 patients (18%) discontinued treatment due to TRAEs. No treatment-related deaths occurred. G-CSF was received by 7 patients (18%) for primary prophylaxis and 10 (26%) for secondary prophylaxis.
Dr. Petrylak concluded his talk as follows:
- In platinum-ineligible patients with metastatic urothelial carcinoma who progressed following checkpoint inhibitor therapy, an ORR of 32% was observed in all patients. Chemotherapy/EV naïve patients had an ORR of 53.8%. The clinical benefit rate was 42%.
- At 9.3 months of median follow-up, the median PFS was 5.6 months, and the median OS was 13.5 months
- SG has a manageable safety profile with no new safety signals and no treatment-related deaths
- Data support further evaluation of SC (alone and in combination) in patients with metastatic urothelial cancer who progressed after prior checkpoint inhibitor therapy
- Cohorts 4, 5, and 6 for 1st line mUC remain open and are currently enrolling
- The TROPiCS-04 phase 3 randomized trial of SG versus single agent chemotherapy of physician’s choice after progression after prior platinum-based and checkpoint inhibitor therapies is ongoing (NCT04527991).
Presented by: Daniel P. Petrylak, MD, Professor of Medicine and Urology, Head of Prostate Medical Oncology, Yale University Cancer Center, New Haven, CT
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 Genitourinary (GU) American Society of Clinical Oncology (ASCO) Annual Meeting, San Francisco, Thurs, Feb 16 – Sat, Feb 18, 2023.
References:
- Koshkin V, et al. Transl Androl Urol. 2021;10:4022-4035
- Tagawa ST, et al. TROPHY-U-01: A Phase II Open-Label Study of Sacituzumab Govitecan in Patients With Metastatic Urothelial Carcinoma Progressing After Platinum-Based Chemotherapy and Checkpoint Inhibitors. J Clin Oncol. 2021;39:2474-2485.
- TRODELVY (sacituzumab govitecan) [prescribing information]. Foster City, CA: Gilead Sciences, Inc.; 2022.


