(UroToday.com) On the third day of the American Society for Clinical Oncology (ASCO) Genitourinary Cancer Symposium 2023, Poster Session focused on Renal Cell Cancer, Adrenal, Penile, Urethral and Testicular Cancers. In this context, Dr. Anand Sharma presented an interim analysis of the CARINA study, a non-interventional study of treatment sequencing and outcomes in patients with advanced renal cell carcinoma (aRCC) initiated on first-line checkpoint inhibitor-based combination therapy.
Over the past few years, checkpoint inhibitor (CPI)-based combinations have become standard of care as first-line therapy for patients with advanced renal cell carcinoma (aRCC). However, optimal subsequent therapies remain to be determined, to allow for optimal sequencing of aRCC therapies. These authors report an interim analysis of CARINA (NCT04957160), a non-interventional study of treatment sequencing and outcomes in patients with aRCC who received first-line CPI-based combination therapy.
To do so, the authors reviewed electronic prescribing records from participating UK specialist centers to identify adult patients (aged 18 years and older) with aRCC who received 2L therapy after 1L CPI-based combination therapy.

The primary objective was to determine the treatment pathway for patients with aRCC who initiated treatment with a CPI-based combination and received subsequent therapy. After characterizing treatment patterns, the authors secondarily sought to assess treatment duration and objective response rate (ORR). The authors considered primarily the entire enrolled population and additionally, the patient subgroup who received 2L cabozantinib.
At the time of this interim analysis, 129 patients were eligible for inclusion. The mean [SD] age at diagnosis of this cohort was 60.0 [9.9] years. Additionally, 75.2% of the cohort was male and 77.3% had clear-cell aRCC histology.
Among the entire enrolled population, 107 (82.9%) received 1L ipilimumab + nivolumab and 22 (17.1%) received axitinib + avelumab. The median (95% CI) duration of 1L treatment was 10.2 (9.1–17.1) weeks and the ORR was 18.4%.
The most commonly prescribed 2L therapies after nivolumab and ipilimumab were cabozantinib (74.8%) and sunitinib (13.1%). Conversely, the most commonly prescribed 2L therapies after axitinib and avelumab were lenvatinib + everolimus (45.5%) and cabozantinib (31.8%).
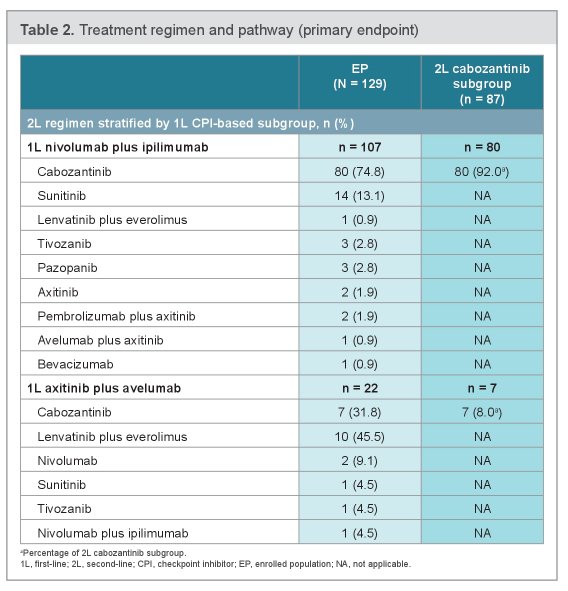
The median (95% CI) duration of all 2L therapies was 23.6 (14.0–28.3) weeks, and median duration of 2L cabozantinib was 28.1 (20.1–37.1) weeks. The ORR to 2L therapy was 30.4% in the entire population and 38.8% among the subgroup of patients who received 2L cabozantinib.

The authors, therefore, concluded that this interim analysis of the CARINA study demonstrated that the most commonly prescribed 2L therapies were cabozantinib and sunitinib after nivolumab and ipilimumab and lenvatinib + everolimus and cabozantinib after axitinib and avelumab.
Presented by: Anand Sharma, MD, D.Phil, MRCP, MBBS, Mount Vernon Cancer Centre, East and North Hertfordshire NHS Trust

