(UroToday.com) On the third day of the American Society for Clinical Oncology (ASCO) Genitourinary Cancer Symposium 2023 focussing on kidney cancer, the Rapid Abstract Session emphasized Biomarkers of Response and Risk Stratification in Genitourinary Cancers. In this context, Dr. Yann-Alexandre Vano presented overall survival and efficacy results from the randomized phase II BIONIKK trial of second-line treatment for patients with metastatic clear cell renal cell carcinoma (mRCC).
While there has been a rapid proliferation of treatment options for patients with mRCC over the past few years, thus far, there have been no biomarkers of efficacy to guide treatment selection between nivolumab+/-ipilimumab (N+/-I) or anti-VEGFR TKI that have been prospectively validated in this disease space. In the previously presented analysis, the BIONIKK trial showed promising objective response rate (ORR) and progression-free survival (PFS) with these treatments in first line (L1) after selection by tumour molecular group. In this abstract, the authors provide updated data assessing overall survival and efficacy results according to second-line (L2) treatment.
As previously reported, BIONIKK is a French multicentre non-comparative phase II trial (NCT02960906), randomising 199 patients with mRCC to receive N (58), NI (101) or TKI (40) in L1 according to four molecular groups (ccrcc1-4).

In prior reports, ORR and PFS have been presented. These results demonstrated that both nivolumab and nivolumab and ipilimumab had higher ORR and median PFS in ccrcc4 than in ccrcc1. Further, regardless of molecular group, nivolumab and ipilimumab had higher ORR and median PFS than nivolumab monotherapy. Additionally, in patients in the ccrcc2 subgroup, VEGF-TKI provided the highest ORR and median PFS.
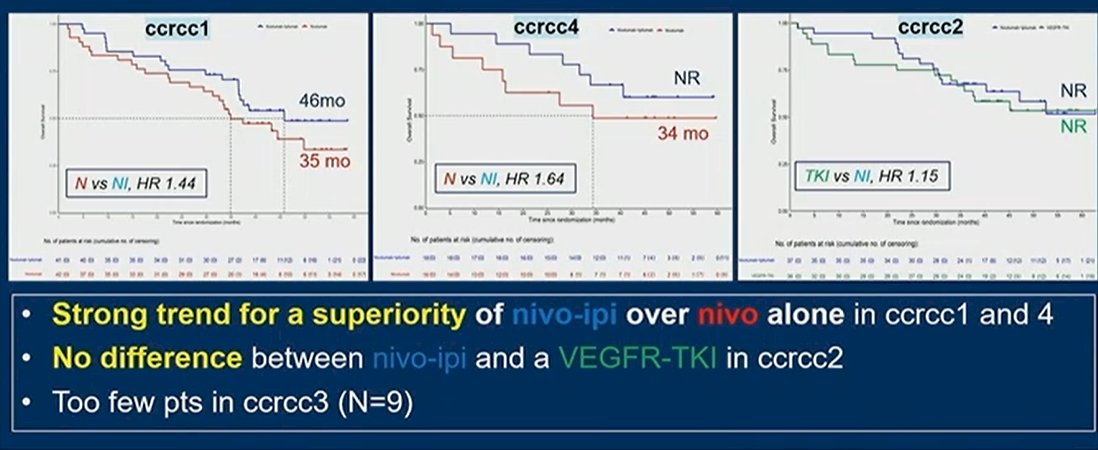
Thus, relying on additional follow-up, these authors report from randomization and from the start of L2, as well as ORR and PFS with a TKI in L2 by molecular group. With maturation of data leading to a median follow-up of 42.1 months (40.5-45.2), 86 (43%) patients died, of whom 27 (of 58, 46.5%) were treated with nivolumab, 39 (of 101, 39%) were treated with nivolumab and ipilimumab and 20 (of 40, 50%) received TKI. The median OS was 34.9 months (95%CI=15.8-NR) for patients treated with nivolumab, NR months (95%CI=29.8-NR) for patients treated with nivolumab and ipilimumab, and 45.2 months (95%CI=14.1-NR) for those treated with a TKI.

When stratified by molecular subgroup, there was evidence of differential efficacy of nivolumab and ipilimumab compared to nivolumab monotherapy in patients with ccrcc1 and ccrcc4 without meaningful differences in ccrcc2.
Among the 199 enrolled patients, 175 (88%) patients discontinued first-line treatment, including 20 deaths. Of the 175 patients who discontinued first-line therapy, 129 (74%) received a second-line therapy, of whom 38 (of 58, 65.5%) following nivolumab, 64 (of 101, 63%) following nivolumab and ipilimumab, and 27 (of 40, 67.5%) following TKI.
The most frequent second-line therapy received after nivolumab with or without ipilimumab was a TKI in 96/102 (94%) patients, including cabozantinib in 49, sunitinib or pazopanib in 32, axitinib in 13, and lenvatinib in 2. Following initial TKI, nivolumab was the most frequent second-line therapy (20 of 27, 74%). In the second-line space, the ORR with TKI was 27% after nivolumab, 45% after nivolumab and ipilimumab and 57% after TKI, with a particular benefit in patients in the ccrcc2 subset.

In second-line therapy, the mPFS with TKI was 8.7 after nivolumab, 11.4 after nivolumab and ipilimumab, and 12 months after TKI, with a higher benefit in patients with ccrcc2. Conversely, ORR and mPFS with nivolumab after TKI in patients in the ccrcc2 group were 12.5% (2/16) and 5.4 (2.6-NR) months, respectively.
In conclusion, the authors note that this is the first-report of first-line overall survival and second-line efficacy result, stratified by molecular group in a randomized trial. These data demonstrate that molecular selection also has an impact on treatment efficacy in the second-line setting. They further note that there is a high efficacy of the combination of nivolumab and ipilimumab in patients with ccrcc4 and ccrcc2 tumors, though ccrcc2 appear to also be sensitive to VEGF-TKI monotherapy as well.
Presented by: Yann-Alexandre Vano, MD, PhD; Department of Medical Oncology, Hôpital Européen Georges Pompidou, Institut du Cancer Paris CARPEM, AP-HP.Centre – Université Paris Cité
Related Content:
Overall Survival and Efficacy Results of Second-line Treatment for Patients With Metastatic Clear Cell Renal Cell Carcinoma, BIONIKK Trial -Yann Vano


