(UroToday.com) The 2023 American Society of Clinical Oncology Genitourinary (ASCO GU) cancers symposium held in San Francisco, CA between February 16th and 18th was host to a Renal Cell Cancer; Adrenal, Penile, Urethral and Testicular Cancers poster session. Dr. Toni Choueiri presented subgroup analysis of the KEYNOTE-564 trial of adjuvant pembrolizumab for renal cell carcinoma across the UCLA Integrated Staging System (UISS) risk groups and disease stage.
In the pivotal randomized, placebo-controlled, double-blind phase III KEYNOTE-564 trial (NCT03142334), adjuvant pembrolizumab was shown to significantly prolong disease-free survival (DFS) in patients with clear cell renal cell carcinoma (ccRCC) and high risk of recurrence (tumor stage 2 with nuclear grade 4 or sarcomatoid differentiation, tumor stage 3 or higher, regional lymph-node metastasis, or stage M1 with NED).1 In an updated efficacy analysis with a median follow up of 30.1 months, pembrolizumab continued to demonstrate a DFS benefit compared with placebo (HR: 0.63, 95% CI: 0.50 to 0.80). The KEYNOTE-564 trial enrolled a broad population of patients with various disease characteristics associated with varying risks of RCC recurrence, including American Joint Committee on Cancer (AJCC) primary tumor stage, Fuhrman nuclear grade, and lymph node involvement. The University of California Los Angeles Integrated Staging System (UISS) for RC is a validated prognostic model that uses the size of the primary tumor (T), involvement of lymph nodes (N), and presence of metastases (M) staging, Fuhrman nuclear grade, and Eastern Cooperative Oncology Group performance status (ECOG PS) to predict 5-year survival rates following nephrectomy.2
The objective of this analysis was to explore the efficacy of adjuvant pembrolizumab in subgroups of patients with ccRCC enrolled in the KEYNOTE-564 study based on UISS risk groups and disease stage.
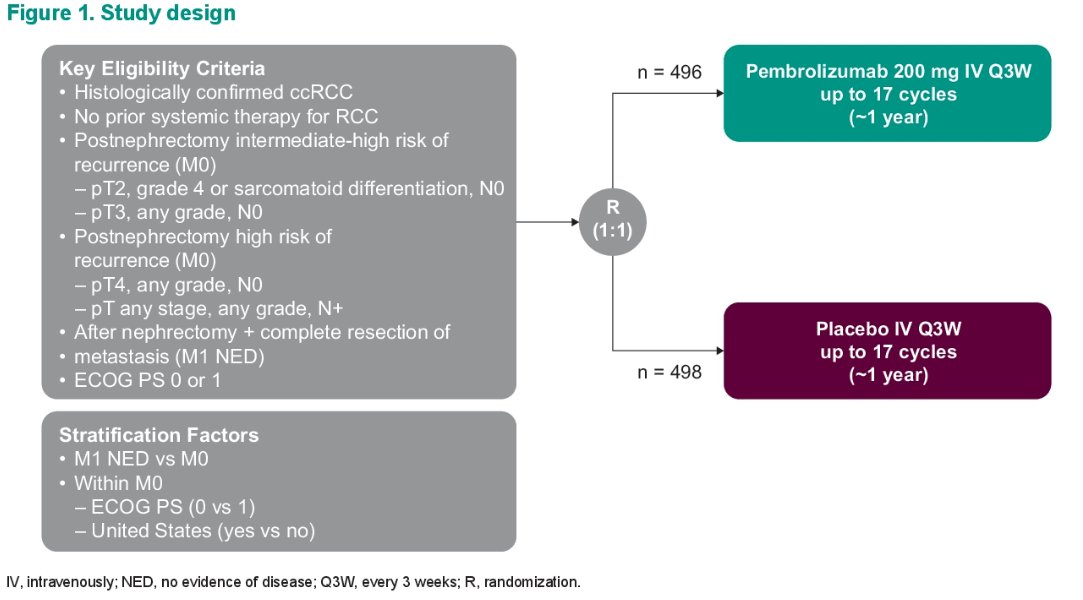
This analysis included 994 patients randomized 1:1 to either pembrolizumab 200 mg IV every 3 weeks up to 17 cycles (~1 year) or placebo (same frequency and number of cycles). UISS risk groups were derived retrospectively.
The post hoc exploratory endpoints were DFS and distant metastasis-free survival (DMFS) in subgroups defined by UISS risk group and TNM disease stage. Kaplan-Meier curves were used to estimate DFS and DMFS. Cox proportional hazards modeling was used to estimate the hazard ratios and 95 confidence intervals, with the Efron method of handling ties used and treatment group defined as the primary exposure of interest. Data cutoff date was June 14, 2021, with a median study follow-up (time from randomization to database cutoff) of 30.1 months (range: 20.8 – 47.5 months).
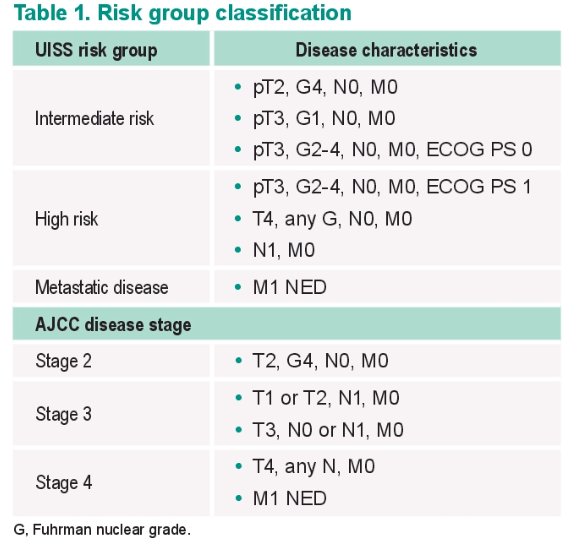
The distribution of patients in each arm by UISS risk category, AJCC disease stage, and TNM status and Fuhrman nuclear grade is demonstrated below. Approximately 75% of patients in each arm were UISS intermediate risk, with 5.9% of the total cohort M1 NED. Almost 88% of patients had AJCC stage 3 disease.
Baseline characteristics are demonstrated below:

Of note, patients with UISS high risk were less likely to have ECOG PS 0 (32.6-34.0% versus 99.2-99.4% for UISS intermediate risk). As per the UISS definition, patients in the UISS high risk group had N1 disease in 26.3 – 29.0% of patients, compared to none in UISS intermediate risk. Baseline characteristics were otherwise well balanced between the two treatment arms.
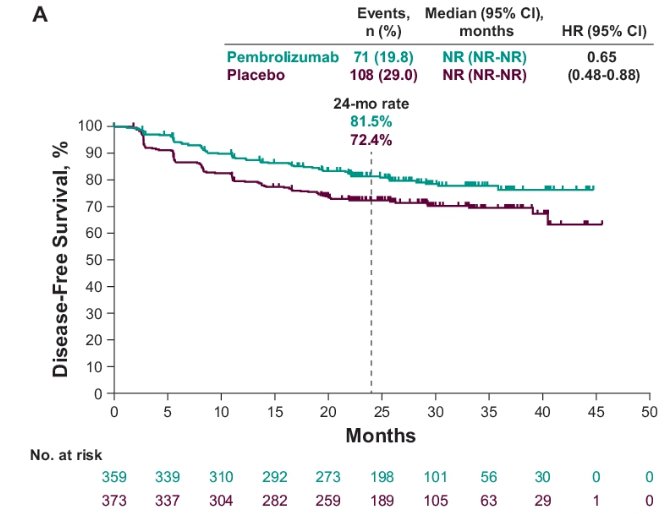
Stratified analysis by UISS risk group demonstrated the following DFS estimates:
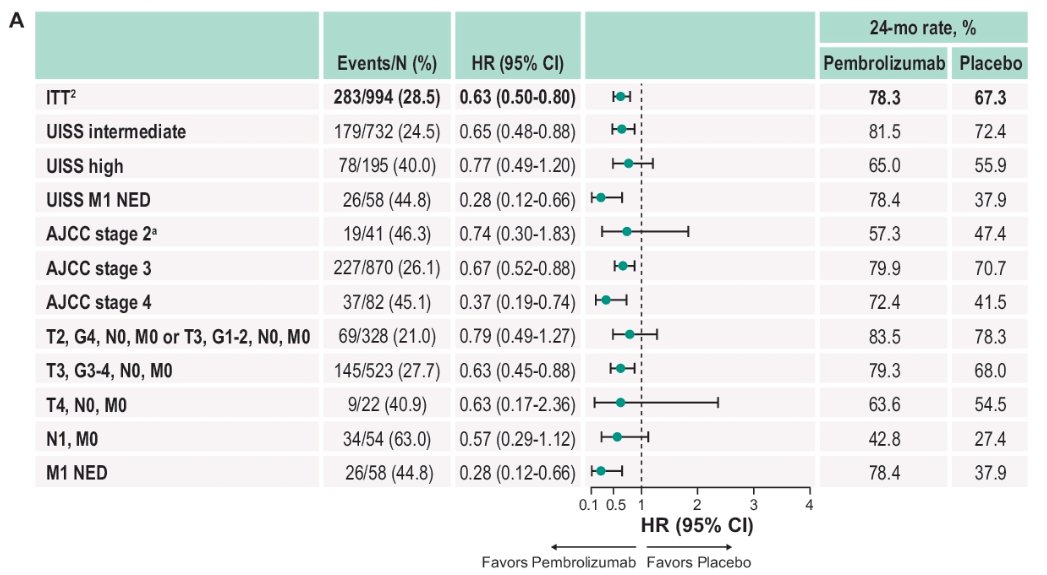
- Intermediate risk: Median NR for pembrolizumab versus NR for placebo (HR: 0.65, 95% CI: 0.48 to 0.88)
- 24-month rate: 81.5% versus 72.4%
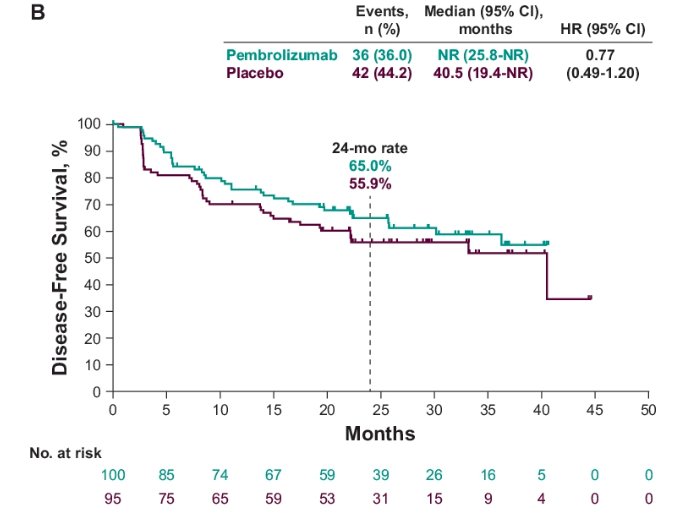
- High risk: Median NR for pembrolizumab versus 40.5 months for placebo (HR: 0.77, 95% CI: 0.49 -1.2)
- 24-month rate: 65% versus 55.9%
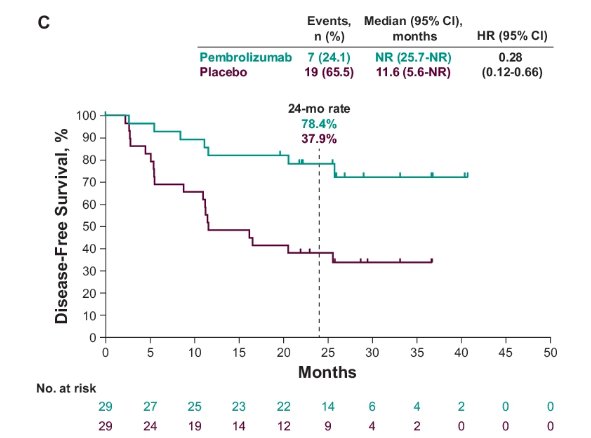
- M1 NED: Median NR for pembrolizumab versus 11.6 months for placebo (HR: 0.28, 95% CI: 0.12 – 0.66).
- 24-month rate: 78.4% versus 37.9%
The forest plot below demonstrates subgroup analysis of DFS:
The forest plot below demonstrates subgroup analysis of DMFS. Similar to DFS, patients in the UISS intermediate (HR: 0.65, 95% CI: 0.47 – 0.88), high (0.79, 95% CI: 0.49 – 1.26), and M1 NED risk subgroups (HR: 0.28, 95% CI: 0.11 – 0.73) all demonstrated DMFS rates in favor of adjuvant pembrolizumab use. The benefit of adjuvant pembrolizumab was most pronounced in patients with AJCC stages 3 (HR: 0.68, 95% CI: 0.51 – 0.89) and 4 (HR: 0.42, 95% CI: 0.20 – 0.87).

Dr. Choueiri concluded as follows:
- Most patients in the pembrolizumab (73.6%) and placebo arms (75.1%) were categorized into the UIS intermediate group
- Adjuvant pembrolizumab prolonged DFS and DMFS compared with placebo across subgroups by UISS risk, AJCC stage, and TNM status and Fuhrman nuclear grade
- The DFS and DMFS benefit across subgroup was consistent with that observed in the ITT population
- No formal statistical testing was conducted for this post hoc analysis, and results should be interpreted with caution due to the small sample size of some subgroups
- Results of this exploratory analysis further support the use of adjuvant pembrolizumab after nephrectomy as standard of care for patients with RCC at increased risk of recurrence.
Presented by: Toni K. Choueiri, MD, Jerome and Nancy Kohlberg Professor of Medicine, Dana-Farber Cancer Institute and Harvard Medical School, Boston, MA
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 Genitourinary (GU) American Society of Clinical Oncology (ASCO) Annual Meeting, San Francisco, Thurs, Feb 16 – Sat, Feb 18, 2023.
References:
- Choueiri TK, et al. Adjuvant Pembrolizumab after Nephrectomy in Renal-Cell Carcinoma. N Engl J Med 2021;385:683-694.
- Zisman A, et al. Risk Group Assessment and Clinical Outcome Algorithm to Predict the Natural History of Patients With Surgically Resected Renal Cell Carcinoma. J Clin Oncol 2002;20(23):4559-66.


