(UroToday.com) In this abstract, Hilser et al. present their retrospective real world evaluation of cabozantinib and nivolumab in adult patients with advanced or metastatic renal cell carcinoma.
At this time, the standard treatment for 1st-line metastatic renal cell carcinoma (mRCC) are IO-combinations. Unfortunately, while trial data is great, data from real-world collectives are rare. In this German multicenter study, the authors evaluated safety and effectiveness of cabozantinib/nivolumab in a real world setting.
Data was collected retrospectively from eight GU cancer centers in Germany. Patients (pts) with advanced or metastatic renal cell carcinoma (mRCC) were eligible. Treatment with cabozantinib 40 mg orally + nivolumab 240 or 480 mg i.v. was mandatory and administered according to routine care. Adverse events (AEs) were reported according to CTCAE 5.0. Objective response rate per RECIST 1.1 and Progression Free Survival (PFS) were calculated from start of treatment to progression or death. Descriptive statistics and KM-plots were utilized, where appropriate.
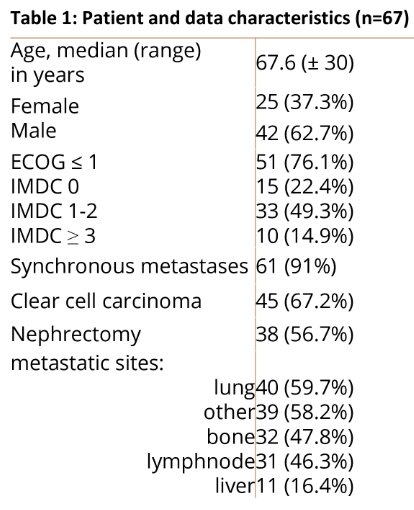
67 suitable pts (62.7% male) with median age of 67.6 years were included. The most common histology was clear cell RCC (ccRCC) in 67.2% (n=45). Nephrectomy was performed in 56.7% (n=38). ECOG 0-1 was 76.1% (n=51). IMDC scores were: 0 in 11 (16.4%), ≥ 1 in 45 (67.1%), missing in 11 pts (16.4%). 29.9% (n=20) required dose reductions or interruptions.
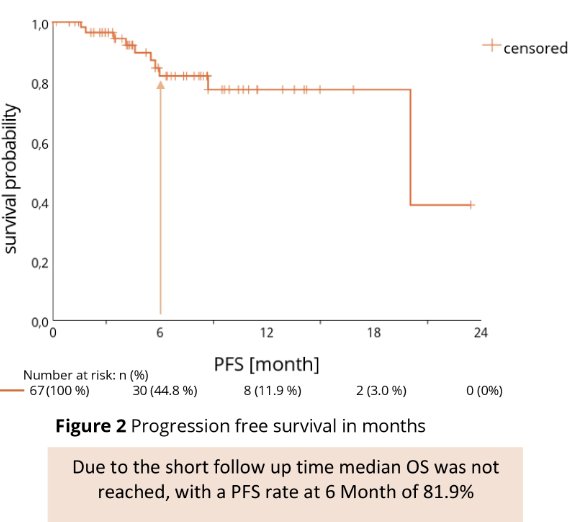
In terms on oncologic response, partial response was documented in 46.3% (n=31), stable disease in 32.8% (n=22), and progressive disease in 4.5% (n=3) as best overall response. Data were missing in 14.9% (n=10). Median Follow-up was 8.3 mo, median treatment duration was 6.0 months, PFS rate at 6 month was 81.9% overall (79.3% for ccRCC; 85.9% for non-ccRCC).
With regards to safety, AEs (all grades) were reported in 82.1% (n=55) and 47.8% (n=32) for grade 3-5. Elevated liver enzymes (40.3%), diarrhea (22.4%) and hand-foot-syndrome (20.9%) were the 3 most frequent AEs of any grade and causality.
They therefore concluded that the cabo/nivo combination was safe and feasible in the real-world setting, with no new safety signals. However, dose reductions were frequently required.
Presented by: Thomas Hilser, MD, University Hospital Essen, Essen, Germany
Written by: Thenappan (Thenu) Chandrasekar, MD – Urologic Oncologist, Associate Professor of Urology, University of California, Davis @tchandra_uromd on Twitter during the 2023 Genitourinary (GU) American Society of Clinical Oncology (ASCO) Annual Meeting, San Francisco, Thurs, Feb 16 – Sat, Feb 18, 2023.


