(UroToday.com) In this abstract, Dr. Feldman presents a trial in progress, STARLITE 2, which is a phase 2 study of nivolumab plus 177lutetium-labeled anti–carbonic anhydrase IX (CAIX) monoclonal antibody girentuximab (177Lu-girentuximab) in patients with advanced clear cell renal cell carcinoma (ccRCC).
As background, CAIX is a cell surface glycoprotein expressed in >90% of ccRCC but rarely in normal tissues, providing a target for imaging and therapeutic application. Radiolabeling the anti-CAIX monoclonal antibody girentuximab with 89Zr has shown promise as a novel PET tracer and labeling with 177Lu promise as a therapeutic agent in ccRCC.
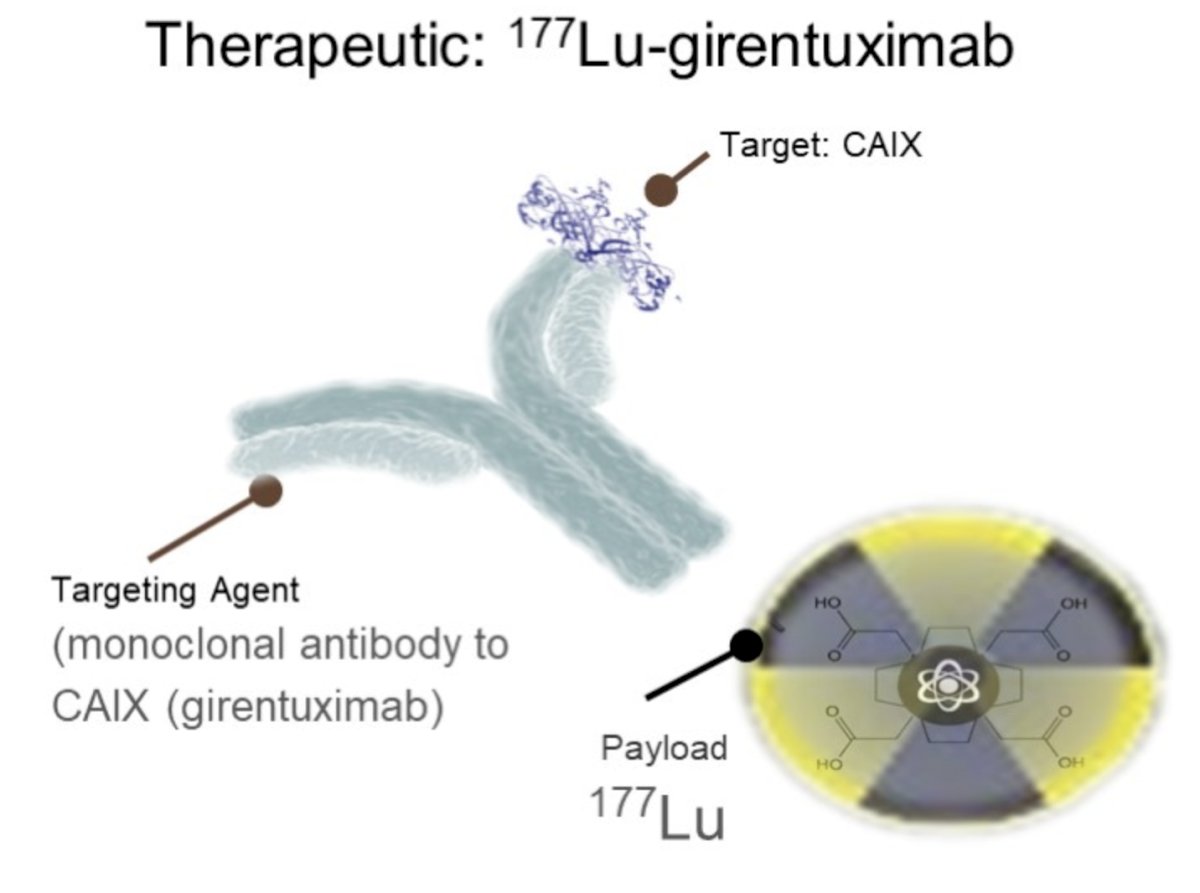
Targeted delivery of radiation to ccRCC cells may prime the immune response by enhancing tumor antigen presentation, providing rationale for combining 177Lu-girentuximab with the anti-PD-1 antibody, nivolumab.
STARLITE 2 is a phase 2, open-label, single arm study (NCT05239533) that is being conducted to evaluate 177Lu-girentuximab in combination with nivolumab in patients with previously treated ccRCC.
The Study Design is summarized in the diagram below:
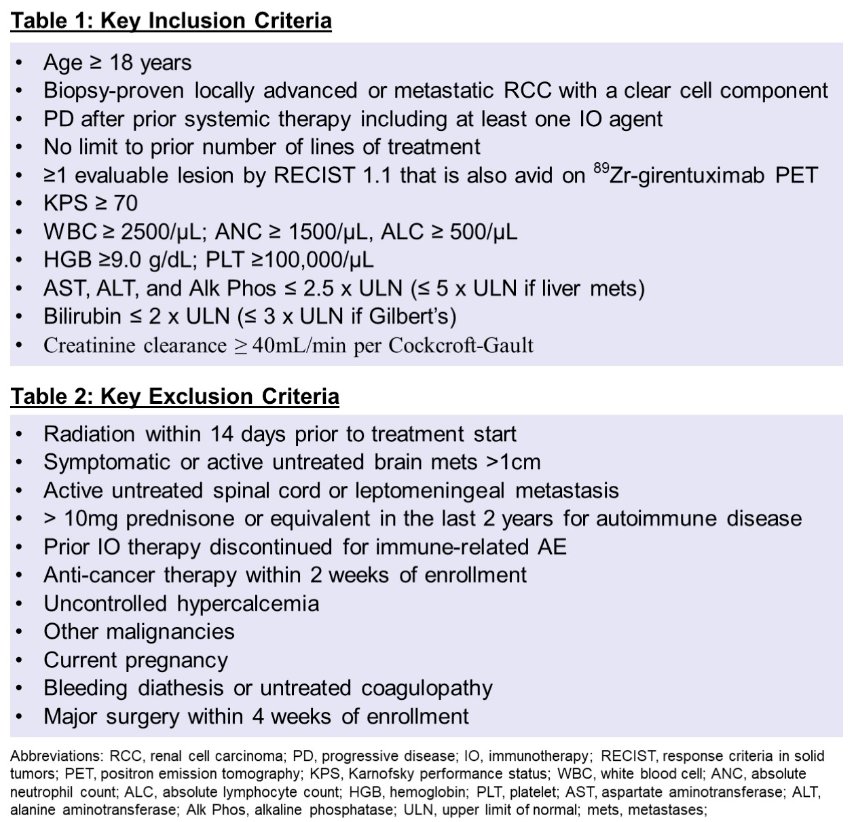
Inclusion criteria are: Pts with biopsy-proven ccRCC, progressive disease after prior systemic therapy including ≥1 immunotherapy (IO) agent, adequate organ/marrow function, and ≥1 evaluable lesion by RECIST 1.1 that is also avid on 89Zr-girentuximab PET will be enrolled.
- There is no limit on number of prior lines of systemic therapy, but pts who stopped IO for immune toxicity are excluded.
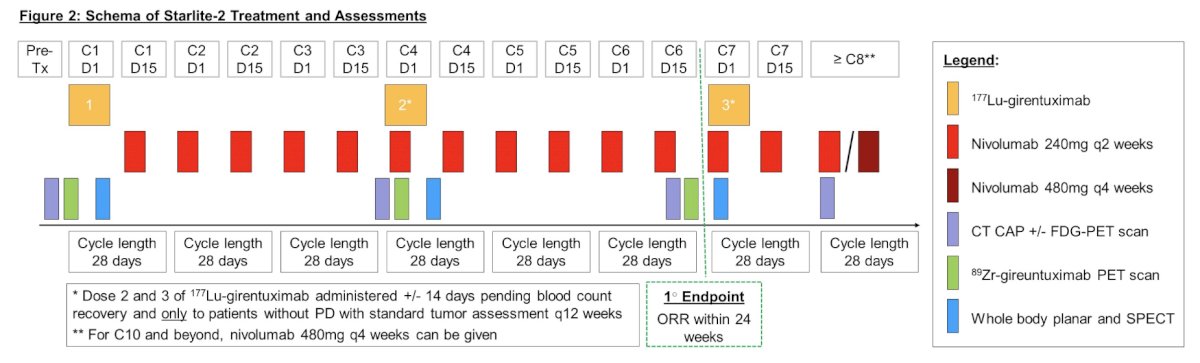
Treatment consists of 177Lu-girentuximab every 12-14 weeks for a maximum of 3 doses plus nivolumab 240mg every 2 weeks until progressive disease (PD) or unacceptable toxicity. Due to expected cumulative myelosuppression, each subsequent 177Lu-girentuximab dose to the same patient is reduced by 25% (dose 2 = 75% of dose 1; dose 3 = 75% of dose 2). Tumor imaging is performed every 12 weeks.
Exploratory imaging with 89Zr-girentuximab PET is performed at baseline and before each 177Lu-girentuximab dose with results correlated with RECIST response on conventional imaging. In addition, whole body planar and SPECT imaging are performed after each 177Lu-girentuximab dose to evaluate distribution, lesion uptake and dosimetry of 177Lu-girentuximab.
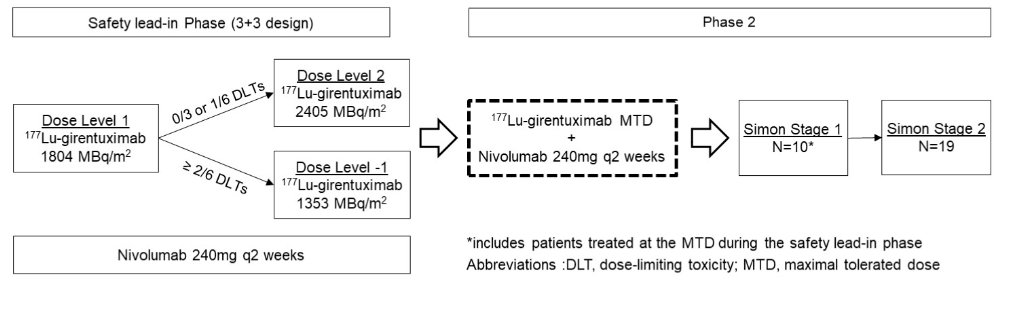
Patients will be evaluated in a safety lead-in phase followed by an expansion phase.
In the safety lead-in phase, the MTD of 177Lu-girentuximab in combination with nivolumab will be determined with a 3+3 design using a starting dose of 1804 MBq/m2 (75% of single agent MTD). For cohort 2, dose escalation to 2405 MBq/m2 (single agent MTD) or de-escalation to 1353 MBq/m2 will be based on dose-limiting toxicities.
In the expansion phase, a Simon 2-stage optimal design is used to evaluate the primary endpoint of response rate by RECIST 1.1 within 24 weeks. With ≥1 response in the first Simon stage (n=10; includes pts treated at MTD in the safety lead-in phase), a second stage will open (n=19) for a total of 29 pts with ≥3 responses indicating the regimen worthy of further study.
Secondary endpoints include PFS, OS, and toxicity including a continuous safety monitoring rule during expansion.
At this time, the trial is currently accruing to the safety lead-in phase.
Presented by: Darren R. Feldman, MD, Memorial Sloan Kettering Cancer Center
Written by: Thenappan (Thenu) Chandrasekar, MD – Urologic Oncologist, Associate Professor of Urology, University of California, Davis @tchandra_uromd on Twitter during the 2023 Genitourinary (GU) American Society of Clinical Oncology (ASCO) Annual Meeting, San Francisco, Thurs, Feb 16 – Sat, Feb 18, 2023.


