(UroToday.com) The 2023 GU ASCO annual meeting included a session on renal cell carcinoma (RCC), featuring a presentation by Dr. Yoshihiko Tomita discussing an exploratory analysis from JAVELIN Renal 101, specifically assessing C-reactive protein as a predictive marker for outcomes with avelumab + axitinib in patients with poor-risk advanced RCC. Analyses from the phase 3 JAVELIN Renal 101 trial1 (NCT02684006) suggested that CRP levels at baseline and early after treatment may predict outcomes with avelumab + axitinib in patients with advanced RCC. In addition, many patients with International Metastatic RCC Database Consortium (IMDC) poor risk who had prolonged progression-free survival (PFS) and overall survival (OS) were observed at the third interim analysis of OS. Dr. Tomita and colleagues analyzed the association between CRP levels and prolonged PFS/OS with avelumab + axitinib in patients with IMDC poor risk.
CRP levels were assessed at screening and on day 1 of each 6-week cycle. Patients in the avelumab + axitinib arm with 3 or 4-6 IMDC risk factors were categorized into subgroups with CRP normal (baseline CRP <10 mg/L), normalized (baseline CRP ≥10 mg/L and ≥1 CRP value decreased to <10 mg/L during 6-week treatment), or non-normalized (CRP ≥10 mg/L at baseline and during 6-week treatment). CRP levels were compared in patients with prolonged PFS/OS (PFS ≥24 months and OS ≥30 months) or PFS <24 months (any OS duration).
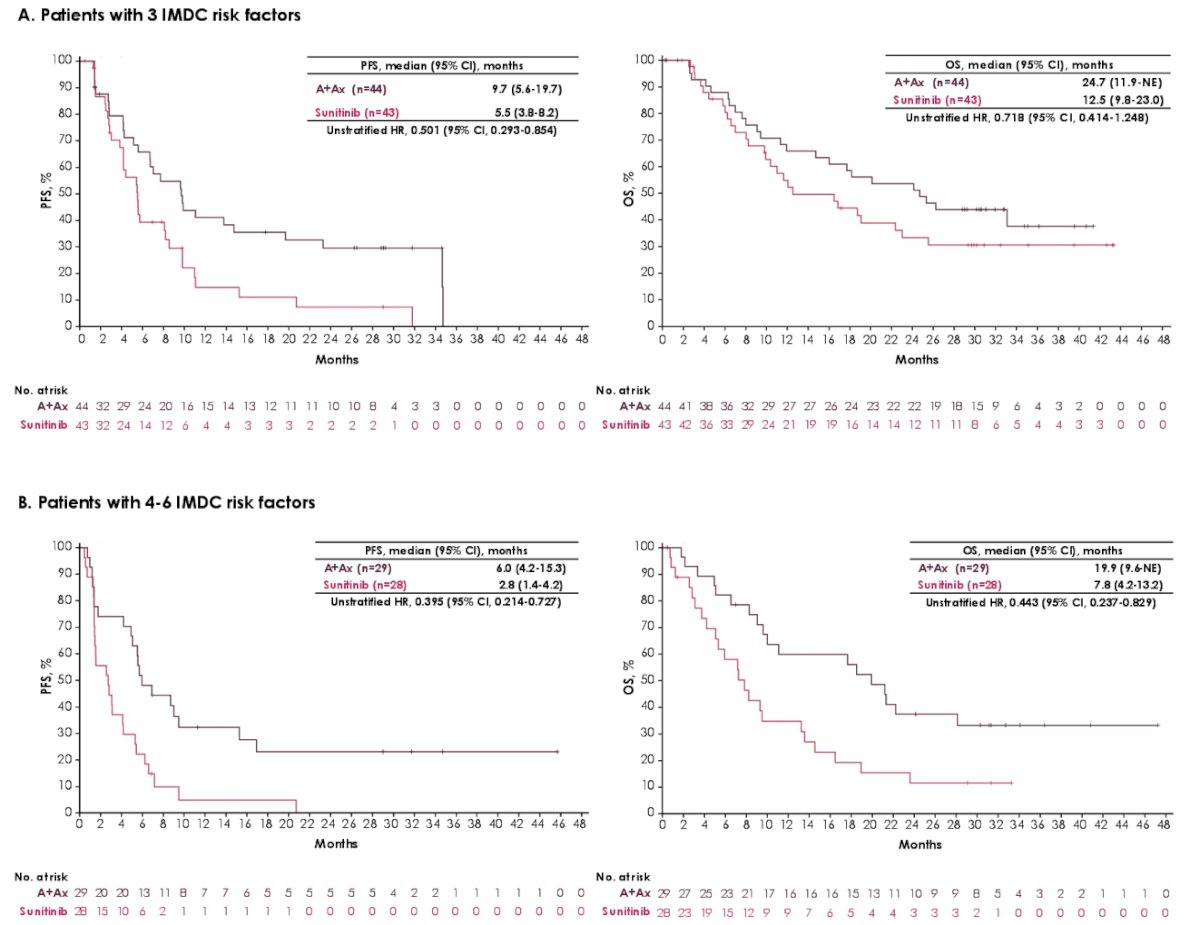
At the data cutoff of April 2020, the minimum follow-up was 28 months. In the avelumab + axitinib arm (n = 442) and in the sunitinib arm (n = 444), 44 and 43 patients had 3 IMDC risk factors, respectively, and 29 and 28 patients had 4-6 IMDC risk factors. PFS and OS favored avelumab + axitinib over sunitinib in patients with 3 or 4-6 IMDC risk factors:
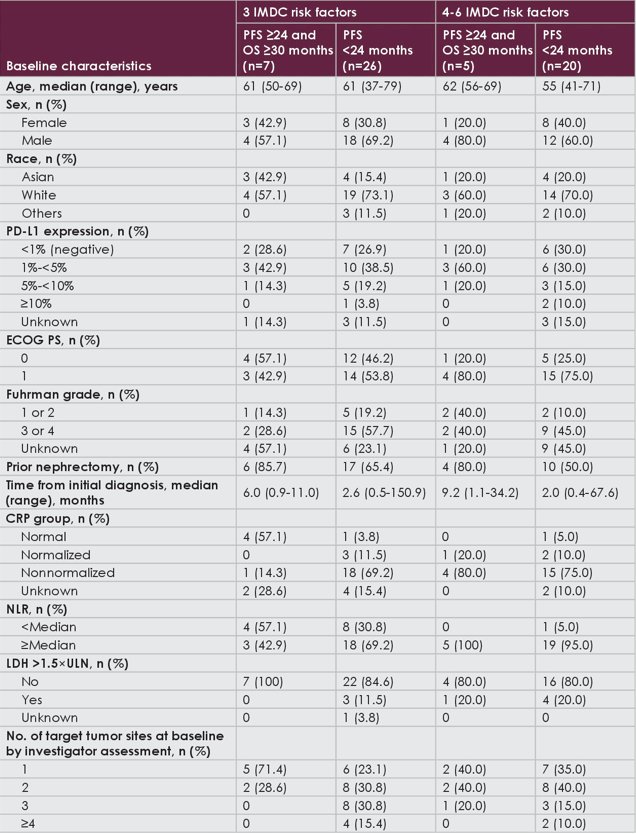
Baseline characteristics of patients in the avelumab + axitinib arm who had prolonged PFS and OS or PFS <24 months is as follows:
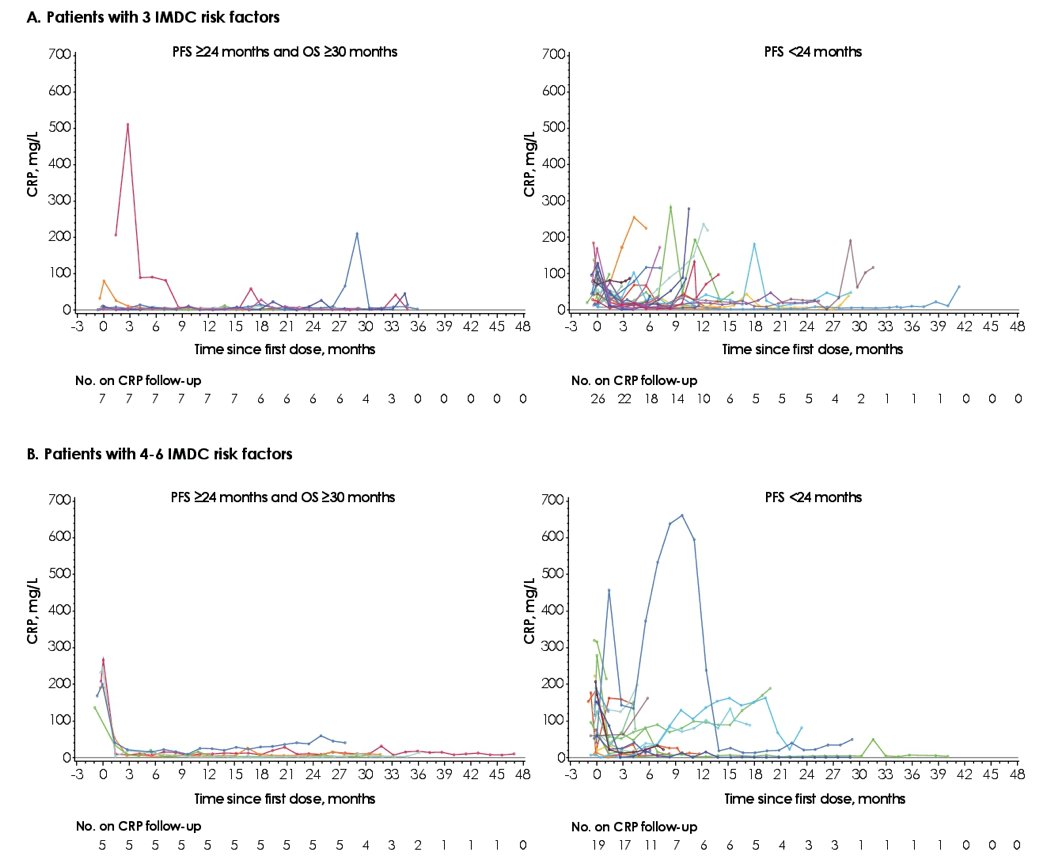
In patients with 3 IMDC risk factors who had prolonged PFS and OS, CRP levels were generally low at baseline and remained low for 24 months. In patients with 4-6 IMDC risk factors who had prolonged PFS and OS, CRP levels were high at baseline but decreased markedly within 6 weeks and were maintained for 24 months:
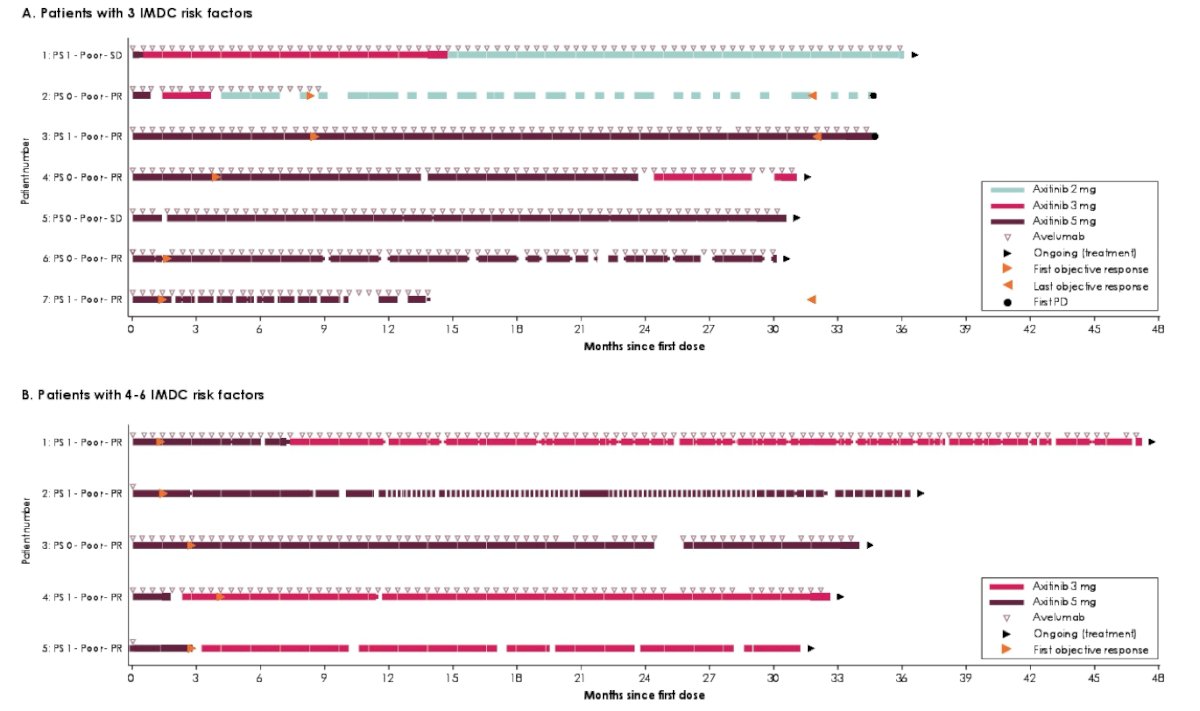
Durable responses were observed in patients with 3 or 4-6 IMDC risk factors who had prolonged PFS and OS:
In patients with poor IMDC risk, PFS and OS generally favored avelumab + axitinib over sunitinib across subgroups, including CRP.
Dr. Tomita concluded this presentation discussing an exploratory analysis from JAVELIN Renal 101, specifically assessing C-reactive protein as a predictive marker for outcomes with avelumab + axitinib in patients with poor-risk advanced RCC with the following concluding messages:
- In the JAVELIN Renal 101 trial, several patients in the avelumab + axitinib arm who had poor IMDC risk (3 or 4-6 IMDC risk factors) had durable responses and prolonged PFS and OS
- In patients with 3 IMDC risk factors who had prolonged PFS and OS (n=7), CRP levels were generally low at baseline and remained low for 24 months
- In patients with 4-6 IMDC risk factors who had prolonged PFS and OS (n=5), CRPC levels were high at baseline but decreased markedly within 6 weeks and were maintained at 24 months
- Low CRPC levels at baseline and during treatment, or a rapid decrease in high CRP level, might predict favorable long-term outcomes in patients with poor-risk advance RCC treated with avelumab + axitinib, although CRPC levels are unspecific and may increase or decrease as a result of other diseases or comorbidities
Presented by: Yoshihiko Tomita, MD, PhD, Niigata University Graduate School of Medicine, Niigata City, Japan
Co-Authors: Robert J. Motzer, Toni K. Choueiri, Brian I. Rini, Hirotsugu Uemura, Mototsugu Oya, Laurence Albiges, Yosuke Fujii, Yoshiko Umeyama, Bo Huang, Alessandra Di Pietro, Manuela Schmidinger
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2023 Genitourinary (GU) American Society of Clinical Oncology (ASCO) Annual Meeting, San Francisco, Thurs, Feb 16 – Sat, Feb 18, 2023.
References:


