(UroToday.com)In this abstract, Lai and colleagues present on the impact of belzutifan (HIF2 inhibitor) on burden of surgery and surgical complications in patients with Von Hippel Lindau (VHL).
VHL disease is a rare hereditary condition that causes abnormal tumor growth in multiple organs, including renal cell carcinoma (RCC), central nervous system (CNS) hemangioblastomas (Hb), pancreatic neuroendocrine tumors (pNET), and other tumors. Repeated surgeries are often required due to ongoing tumor recurrences and can lead to complications such as chronic kidney disease, pancreatic insufficiency, and neurological deficit. Belzutifan is a hypoxia-inducible factor inhibitor approved in the US for the treatment of VHL-associated RCC, CNS Hb, or pNET not requiring immediate surgery. FDA approval was based on the LITESPARK-004 phase II trial which demonstated clinically meaningful antitumor activity and durable long-term with respect to RCC and other tumors related to VHL.
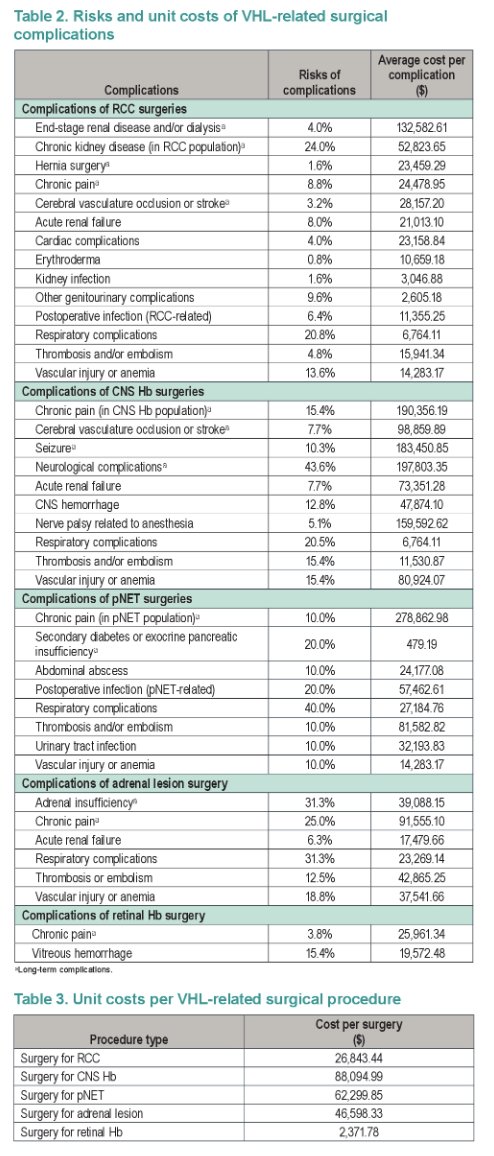
This study aimed to quantify the annual rate and costs of VHL-related surgeries and surgical complications before and after belzutifan initiation. Surgeries were observed during the periods before and after belzutifan initiation in the single-arm phase 2 LITESPARK-004 trial (NCT03401788) among adults with VHL-RCC. Rates of RCC surgeries were obtained by fitting exponential survival models to time from treatment initiation to the next renal surgery; and time (looking backward) from treatment initiation to the most recent renal surgery before treatment. Rates of surgeries related to other VHL manifestations were calculated as number of surgeries divided by person-years at risk, using all available post-treatment follow up (151 person-years; median [range] per patient: 29.2 [4.2-37.5] months) and equivalent person-years in the pre-treatment period. Complication risks per surgery and unit costs of surgeries and complications were obtained from a retrospective study on VHL within the Optum Clinformatics Data Mart (2000-2020). Table 2 and 3 puts the model inputs into perspective. Annual per-patient costs of surgeries and complications were estimated in 2020 US dollars over the pre- and post-treatment periods from a US third-party payer perspective (Table 1).
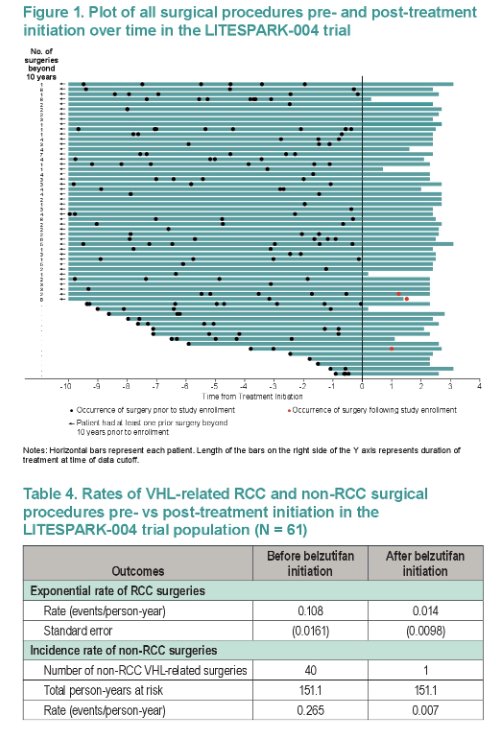
Among trial participants (N=61), the rate of RCC surgeries (surgeries/person-year) decreased 87% (0.108 to 0.014) after belzutifan initiation. The rate of other surgeries decreased 98% (0.265 to 0.007), with CNS Hb surgeries decreasing from 0.185 to 0.007, pNET surgeries from 0.013 to 0, adrenal lesion surgeries from 0.007 to 0, and retinal Hb surgeries from 0.060 to 0.
Belzutifan was accordingly estimated to reduce per-patient average annual costs of surgeries and complications from $57,259 to $2,536.
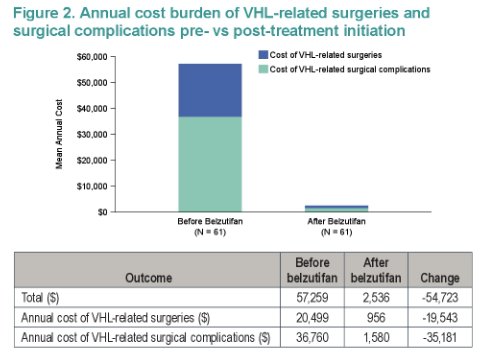
The authors therefore concluded that healthcare costs of surgeries and surgical complications are substantial among patients with VHL-RCC; in a trial-based cost-consequence analysis, belzutifan was estimated to decrease annual surgery and complication costs by 96%, based on observed reductions in VHL-related surgeries.
What wasn’t quite clear was how the cost of the medicine was accounted into the model. Either way, this is a novel therapy for our VHL patients.
Presented by: Yizhen Lai, MSc | Merck & Co., Inc.
Written by: Thenappan (Thenu) Chandrasekar, MD – Urologic Oncologist, Associate Professor of Urology, University of California, Davis @tchandra_uromd on Twitter during the 2023 Genitourinary (GU) American Society of Clinical Oncology (ASCO) Annual Meeting, San Francisco, Thurs, Feb 16 – Sat, Feb 18, 2023.


