(UroToday.com) In this study, Dr. Abdallah and colleagues evaluate the accuracy of fully automated, AI-generated models compared to validated clinical models to predict post-operative GFR after kidney surgery.
As background, the American Urologic Association (AUA) recommends estimation of the postoperative glomerular filtration rate (GFR) in patients with a renal mass to help decide between partial nephrectomy (PN) or radical nephrectomy (RN). If postoperative is estimated to drop below GFR<45 mL/min/1.73m2, a PN should be prioritized.
Most existing methods to predict postoperative GFR are rarely implemented in the clinical setting due to complexity. Previously validated models based on clinical equations or kidney volumes from hand-segmented or semi-automated segmentations are quite accurate but have seen limited uptake in clinical practice.
As such, the current authors hypothesized that they could develop an artificial intelligence (AI)-GFR prediction that would be calculated automatically on a preoperative computed tomography (CT) scan and predict a postoperative GFR as accurately as a validated clinical model.
They included 300 patients undergoing PN or RN for renal tumor from the 2021 Kidney and Kidney Tumor Segmentation Challenge (KiTS21) were analyzed – but they did excluded7 patients having bilateral tumors. Preoperative GFR was the closest recorded value preoperatively while postoperative GFR was measured ≥90 days postoperatively. Split-renal-function (SRF) was determined in a fully automated way from preoperative imaging and their previously developed deep learning segmentation model. They programmed the algorithm to estimate postoperative GFR as 1.24×preoperative GFR×contralateral SRF for RN; and as 89% of the preoperative GFR for PN.
In contrast, they used a validated clinical model (GFR=35+preoperative GFR(x0.65)-18(if radical nephrectomy)-age(x0.25)+3(if tumor size >7 cm)-2 (if diabetes)) as a comparator.
They then compared the AI and clinical model estimations of GFR to the measured postoperative GFR using correlation coefficients (R) and compared the ability of AI models to predict a postoperative GFR<45 using logistic regression and AUCs.
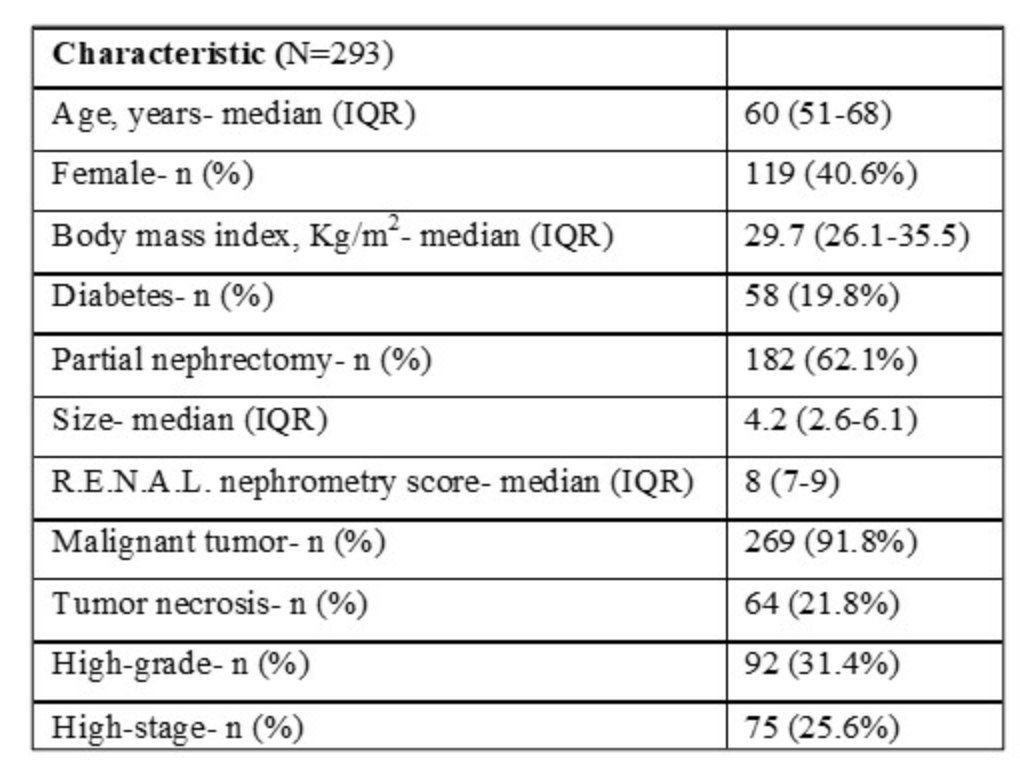
They identified 293 patients. Basic demographic info: median age was 60 years ((IQR) 51-68), 40.6% were female, and 62.1% had PN. The median tumor size was 4.2 (2.6-6.1), and 91.8% of the tumors were malignant, of which 35.1% were high-grade, 25.6% were high-stage, and 21.8% had necrosis. The median R.E.N.A.L. nephrometry score was 8 (7-9).
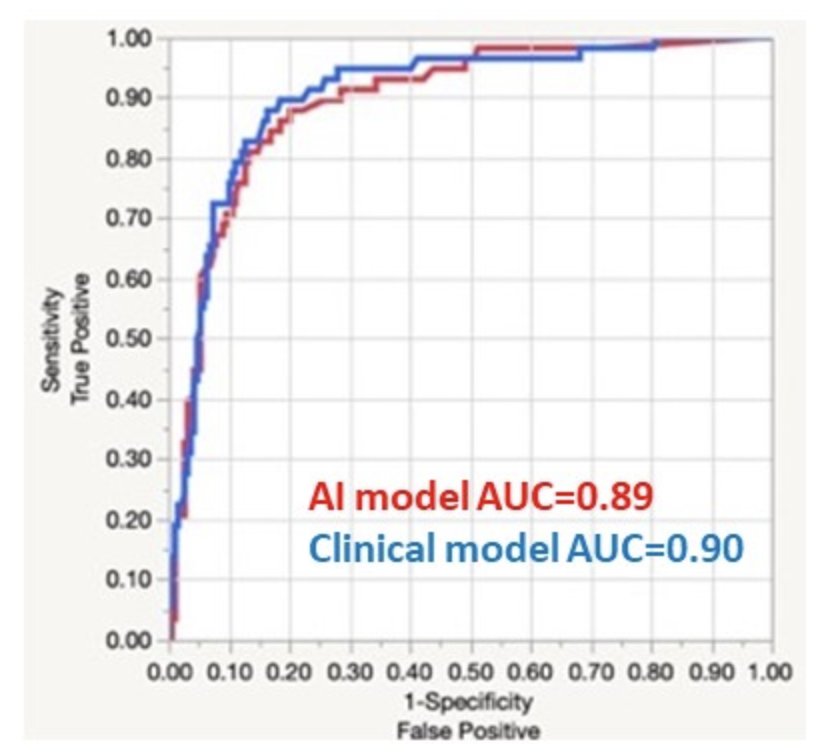
When comparing measured postoperative GFR, the correlation coefficients were 0.75 and 0.77 for the AI model and clinical models, respectively. For the prediction of a postoperative GFR< 45 ml/min/1.73m2, the AI and clinical models performed similarly (AUC of 0.89 and 0.9, respectively).
They demonstrated the feasibility of a fully automated prediction of postoperative GFR based on CT imaging and baseline GFR with comparable predictive accuracy to existing validated clinical prediction models. Importantly, these AI-generated predictions can be implemented for decision-making, with no clinical details, clinician time, or measurements needed. This is key in increasing utilization.
Presented by: Nour Abdallah, MD, Cleveland Clinc
Written by: Thenappan (Thenu) Chandrasekar, MD – Urologic Oncologist, Associate Professor of Urology, University of California, Davis @tchandra_uromd on Twitter during the 2023 Genitourinary (GU) American Society of Clinical Oncology (ASCO) Annual Meeting, San Francisco, Thurs, Feb 16 – Sat, Feb 18, 2023.


