(UroToday.com) The 2023 American Society of Clinical Oncology Genitourinary (ASCO GU) cancers symposium held in San Francisco, CA between February 16th and 18th was host to a Renal Cell Cancer; Adrenal, Penile, Urethral and Testicular Cancers poster session. Dr. Laurence Albiges presented the interim results of CaboPoint, a phase 2 trial of cabozantinib after checkpoint inhibitor therapy in patients with advanced renal cell carcinoma (RCC).
The first line treatment landscape for patients with advanced RCC has rapidly evolved in recent years, with checkpoint inhibitor (CPI)-based combination therapy becoming an established standard of care.1,2 Current guidelines recommend dual CPI combinations (e.g. nivolumab plus ipilimumab) or CPI therapy in combination with vascular endothelial growth factor receptor (VEGFR)-targeting agents (e.g. pembrolizumab plus axitinib or nivolumab plus cabozantinib).3 However, since pivotal 2nd line therapies were established prior to the approval of 1st line CPI-based regimens, there is an emerging need to establish the safety and efficacy of these therapies in the post-CPI setting. The CaboPoint trial (NCT03945773) was designed to address the lack of prospective data for cabozantinib after 1st line CPI regimens. Cabozantinib, a multi-targeted tyrosine kinase inhibitor (TKI), was approved for the 2nd line treatment of advanced RCC following the phase 3 METEOR study, which demonstrated a significant improvement in survival and an ORR of 17% with cabozantinib versus 3% with everolimus following VEGFR-targeted therapy.4
The objective of this study was to evaluate the efficacy and safety of cabozantinib in adults with unresectable, locally advanced, or metastatic RCC with a clear-cell component whose disease progressed after 1st line CPI-based therapy. In this report, Dr. Albiges reported the results of the pre-planned interim analysis, which aimed to describe baseline demographics and characteristics, as well as investigator-assessed ORR at 3 months.
CaboPoint is an ongoing phase II, multicenter, open-label study being conducted at approximately 50 centers across Austria, France, Germany, Netherlands, Spain, Switzerland, and the UK. Eligible patients were adults with histologically confirmed, unresectable, locally advanced or metastatic RCC with a clear-cell component whose disease showed radiographic progression following treatment with a 1st line CPI-based regimen. Prior treatment with cabozantinib was not permitted. Patients received cabozantinib (starting dose 60 mg/day) in two independent cohorts:
- Cohort A: patients with progression after dual CPI therapy (nivolumab plus ipilimumab)
- Cohort B: patients with progression after CPI plus VEGF-targeted therapy
Both cohorts received cabozantinib until study end (18 months after the last patient enrolled had started treatment), but could terminate earlier owing to disease progression, unacceptable toxicity, or withdrawal of consent.

The primary endpoint of the CaboPoint trial was ORR per RECIST v1.1 in cohort A, evaluated by independent central review. Secondary endpoints included:
- ORR in cohort B by independent central review
- ORR for both cohorts by local investigator review
- Time to response
- Duration of response
- Disease control rate
- Progression-free survival (all evaluated by independent central and local investigator review)
- Overall survival
- Change in disease-related symptoms
- Safety
- Tolerability
With regards to sample size calculations, for cohort A: with a true ORR of 23%, a sample size of 68 patients would provide at least 80% power, at a 1-side significance level (alpha) of 0.025 to reject the null hypothesis of 10%. Assuming approximately 7% non-evaluable subjects, up to a total of 74 patients in cohort A will be included in the study. For cohort B, no formal sample size calculation was performed, and enrollment will stop when recruitment in cohort A is reached.
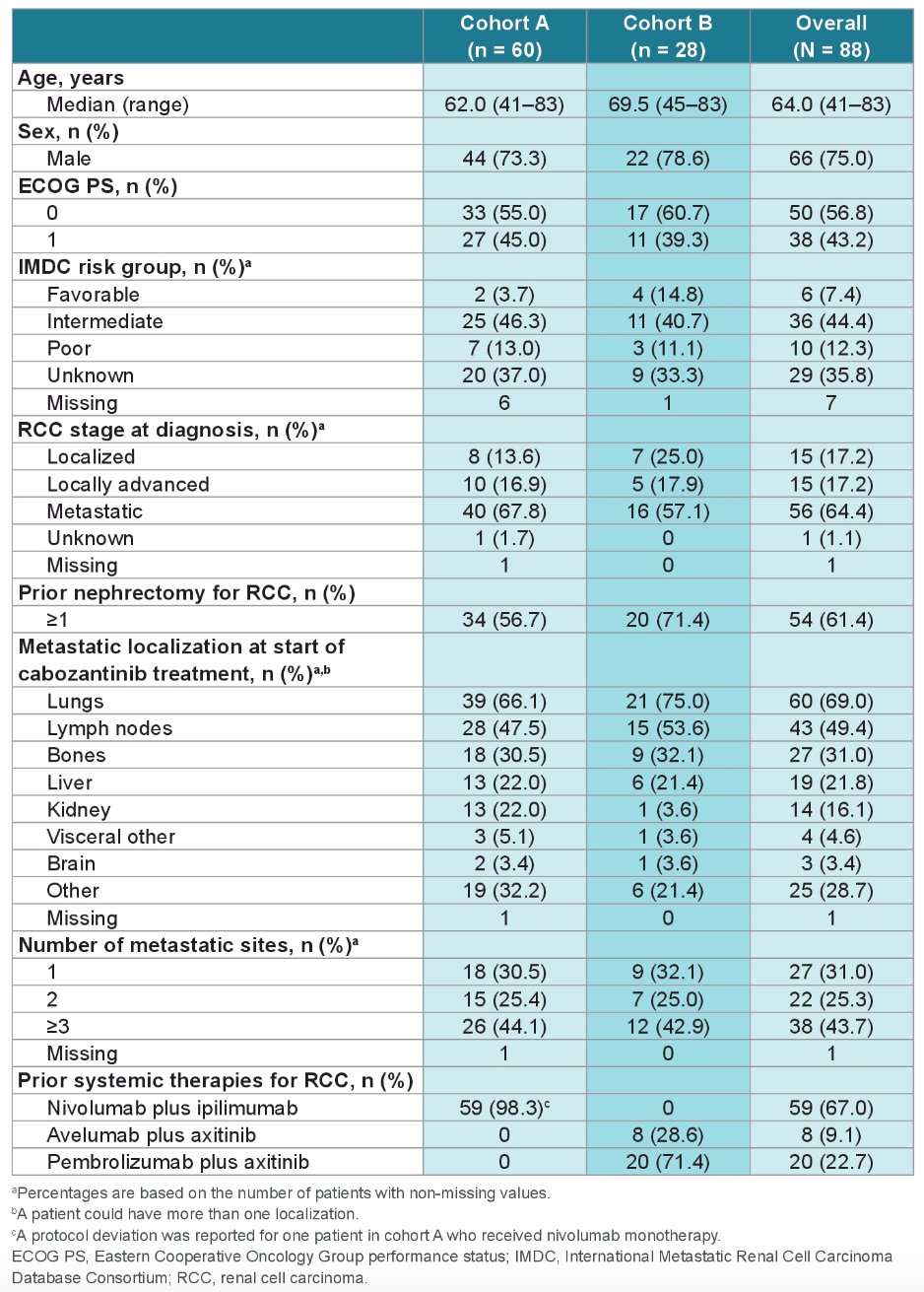
For the interim analysis, this was pre-planned once 80% of patients in cohort A reached at least 3 months of treatment. At the time of this analysis (data cut-off: July 12, 2022), 88 patients had at least 3 months of follow-up (60 in cohort A and 28 in cohort B). Baseline characteristics were similar across both cohorts.
The ORR was 31.7% in cohort A and 25.0% in cohort B. Overall, 1 patient achieve a complete response, and 25 patients experienced partial responses.

Dr. Albiges concluded as follows:
- The CaboPoint trial is the first prospective trial to evaluate the efficacy and safety of cabozantinib following disease progression on 1st line CPI therapy in a large European cohort of patients with locally advanced or metastatic RCC with a clear cell component.
- In this interim analysis, cabozantinib demonstrated preliminary efficacy in patients with advanced RCC after progression on CPI-based combination therapy, irrespective of 1st line regimen.
- The CaboPoint trial is ongoing, with the final analysis anticipated in September 2023.
Presented by: Laurence Albiges MD, PhD, Professor, Medical Oncology, Vice chair of the Department of Cancer Medicine at the Gustave Roussy Institute, Villejuif, France
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, San Francisco, CA, February 16th – February 18th, 2023
References:- Lalani AA, et al. Ther Adv Med Oncol 2022;14:17588359221108685.
- Deleuze A, et al. Int J Mol Sci 2020;21:2532.
- Rathmell WK, et al. J Clin Oncol 2022;40:2957-95.
- Choueiri et al. Lancet Oncol 2016;17:917-27.
CaboPoint Study: Cabozantinib After Checkpoint Inhibitor Therapy in Patients with Advanced Renal Cell Carcinoma - Laurence Albiges


