(UroToday.com) The 2023 American Society of Clinical Oncology Genitourinary (ASCO GU) cancers symposium held in San Francisco, CA between February 16th and 18th was host to a Renal Cell Cancer; Adrenal, Penile, Urethral and Testicular Cancers poster session. Dr. Michael Atkins presented the treatment-free survival (TFS) outcomes of Cohort A from HCRN GU16-260, a phase II trial of nivolumab and salvage nivolumab + ipilimumab in advanced clear cell RCC.
Immunotherapy-based regimens can lead to prolonged disease control after treatment cessation without the need for further systemic antineoplastic therapy. However, treatment-related adverse events can also persist after treatment cessation. TFS can simultaneously characterize disease control and TRAEs for this off treatment period. Significant TFS was reported with immunotherapy in patients with metastatic melanoma or advanced RCC,1,2 but in these studies treatment was halted for toxicity or progression rather than a pre-defined treatment endpoint. The authors thus sought to assess TFS in the HCRN GU16-260 trial, which was designed to reduce toxicity by limiting exposure to CTLA-4 blockade and capping therapy duration at 96 weeks.
This analysis was restricted to patients in cohort A, who all had treatment naïve, metastatic clear cell RCC. The study design is as below, with a treatment escalation approach adopted if there was stable or progressive disease with nivolumab alone. Patients received nivolumab at 240 mg every 2 weeks x6 doses, followed by 360 mg every 3 weeks x4 doses, and then 480 mg every 4 weeks. Patients with a partial or complete response continued nivolumab for up to a total of 96 weeks. Conversely, those with progressive disease or best response as stable disease at 48 weeks, proceeded to part B which involved nivolumab 3 mg/kg plus ipilimumab 1 mg/kg every 3 weeks x4 doses, followed by nivolumab maintenance for up to 48 weeks. TFS begins when treatment stops for TRAE, PD, or treatment completion and ends with start of subsequent therapy or death.
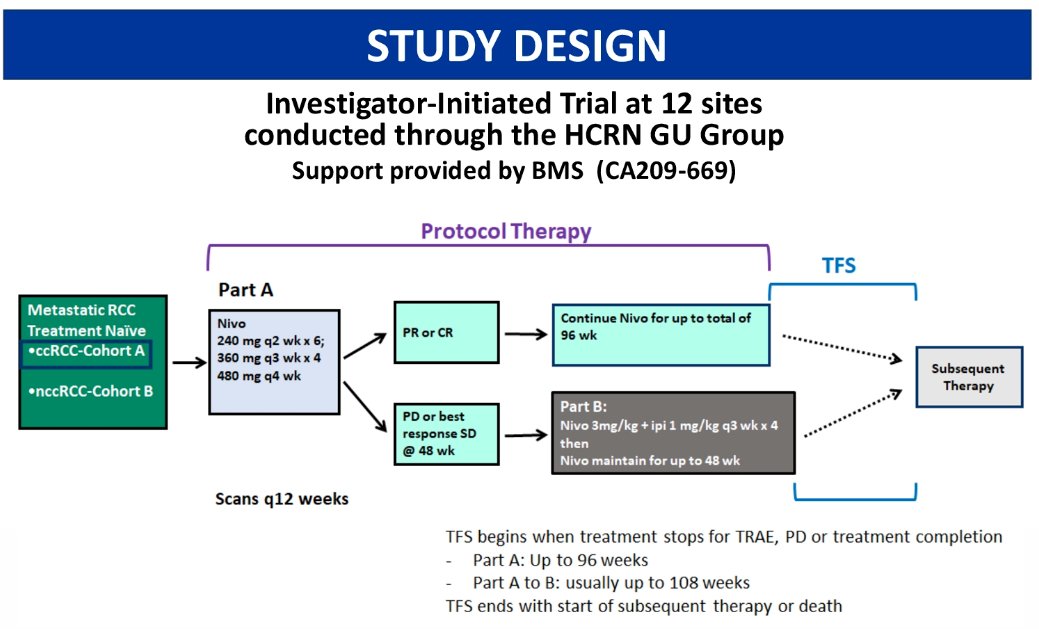
Cohort A included 128 patients. The median age was 65 years (range: 32 – 86), 99% had ECOG PS 0-1, 72% were male, 17% had tumors with sarcomatoid features, and 82% had a prior nephrectomy.
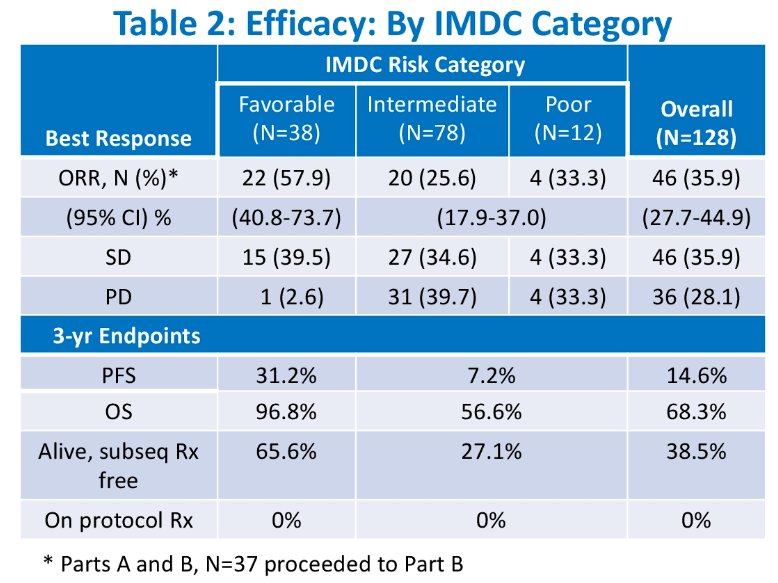
Efficacy results by IMDC category are demonstrated below. At 36 months from enrollment, 68.3% of patients were alive: 96.8% of IMDC favorable-risk pts and 56.6% of those with intermediate/poor-risk, respectively. The ORRs were 58%, 26%, and 33% for patients with IMDC favorable, intermediate, and poor risk categories. With regards to PFS, the following 36-month rates were observed:
- Favorable: 31.2%
- Intermediate: 7.2%
- Poor: 14.6%
For 36-month OS:
- Favorable: 96.8%
- Intermediate: 56.6%
- Poor: 68.3%
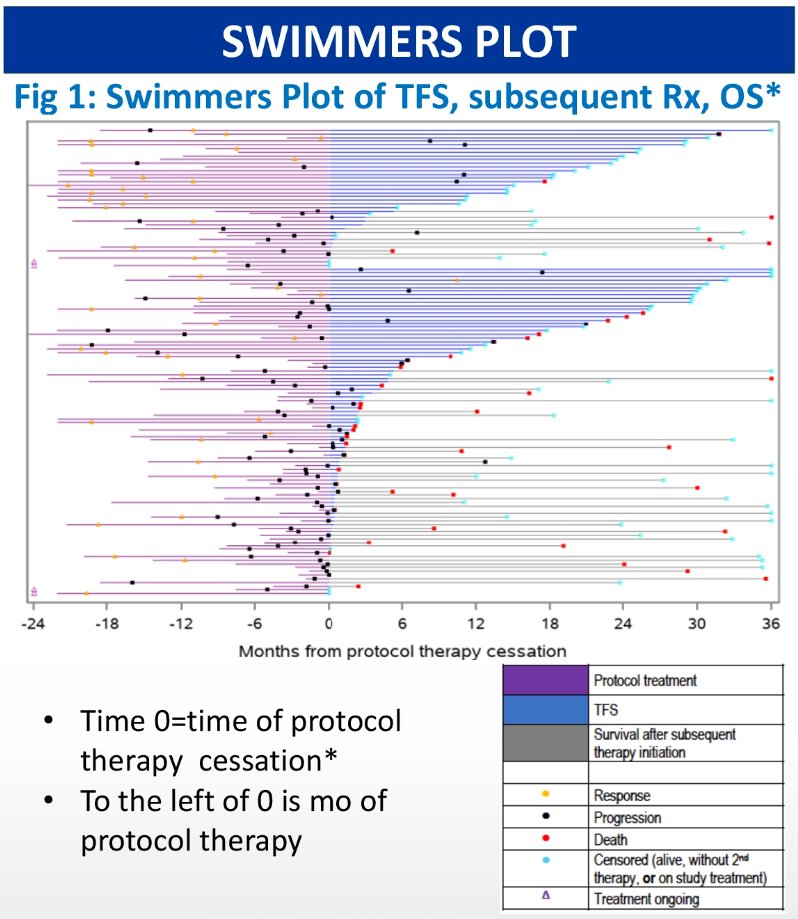
The swimmers plot of TFS, subsequent treatment, and overall survival is demonstrated below:
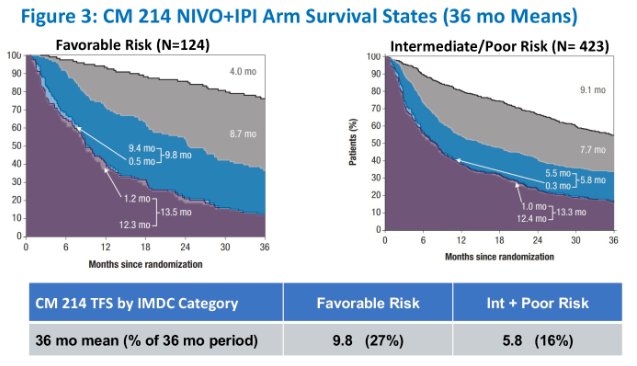
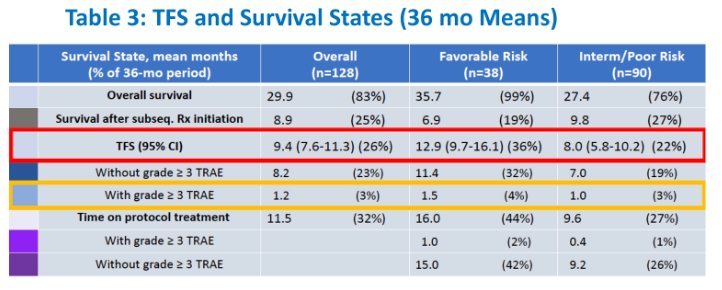
The 36-month mean time on protocol therapy was 11.5 months:
- Favorable: 16.0 months
- Intermediate/poor: 9.6 months
The 36-month mean TFS for the whole population was 9.4 months:
- Favorable: 12.9 months, of which TFS with grade 3+ TRAEs was 1.5 months
- Intermediate/poor: 8.0 months, of which TFS with grade 3+ TRAEs was 1.0 months
At 36 months, 65.6% of favorable risk patients and 27.1% of intermediate/poor risk patients were alive and second-line treatment-free.
Dr. Atkins concluded as follows:
- Nivolumab monotherapy with salvage nivo/ipi in non-responders is an active treatment approach in patients with advanced RCC who are treatment-naïve and results in substantial TFS and TRAE-free TFS
- TFS was increased by, and sustained, following elective treatment cessation at 96-108 weeks
- TFS% over 36 months was greater for HCRN GU16-260 relative to CheckMate 214
- TFS captures valuable information not contained in traditional trial endpoints
- It was particularly notable in patients with favorable disease, further supporting the use of an immunotherapy-only regimen in this population
Presented by: Michael B. Atkins, MD, Deputy Director of the Georgetown Lombardi Comprehensive Cancer Center and the Scholl Professor and vice chair of the Department of Medical Oncology, Georgetown University Medical Center, Washington, DC
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 Genitourinary (GU) American Society of Clinical Oncology (ASCO) Annual Meeting, San Francisco, Thurs, Feb 16 – Sat, Feb 18, 2023.
References:
- Regan et al. JITC 2021.
- Regan et al. Clin Cancer Res 2021.


