(UroToday.com) The 2023 American Society of Clinical Oncology Genitourinary (ASCO GU) cancers symposium held in San Francisco, CA between February 16th and 18th was host to a Renal Cell Cancer; Adrenal, Penile, Urethral and Testicular Cancers poster session. Dr. Jing Liu presented the results of a phase II trial of combination PD-1 immune checkpoint inhibition with all-lesion stereotactic radiation (SBRT) in oligometastatic renal cell carcinoma (RCC).
The five-year survival rates for patients with metastatic RCC remain poor. Through the abscopal effect, SBRT may theoretically improve the antitumor immunity of PD-1 immune checkpoint inhibition in metastatic RCC. Patients with oligometastatic RCC have a low tumor load and favorable prognosis. As such, they represent a prime target for combination SBRT and systemic therapy within the setting of future trials. This study aimed to evaluate whether the antitumor immunity can be enhanced with the combination of immune checkpoint inhibition and all-lesion SBRT for patients with oligometastatic RCC.
This study included patients with progressive disease after ≤2 prior anti-angiogenic therapies and ≤5 lesions located in ≤3 non-brain metastatic sites. All patients in this study received Tislelizumab (anti-PD-1) 200 mg every 3 weeks + SBRT at a dose of 5-8 Gy per fraction for 3 to 10 doses, performed according to lesion site, size, and organs at risk, to all lesions. SBRT started 7 days after the first infusion of Tislelizumab. SBRT of other lesions was performed sequentially during ICI treatment intervals. Among patients who responded (i.e. CR, PR, or SD), Tislelizumab 200 mg every 3 weeks was continued until RECIST v1.1 progression or unacceptable toxicity was encountered.
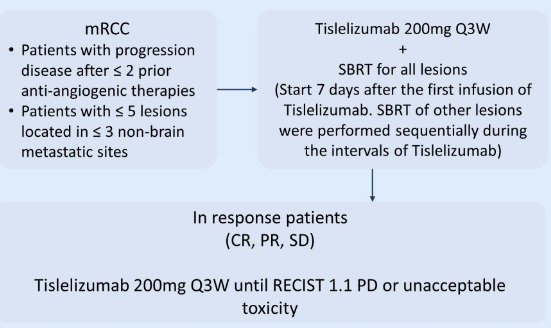
The primary objective was ORR. Secondary endpoints included PFS, OS, and the safety profile. There are plans for subsequent exploratory analyses to evaluate molecular etiologies for the synergistic effects of SBRT and immunotherapy, as well as potential mechanism of immune checkpoint inhibitor resistance.
Fifteen patients were enrolled between May 2021 and May 2022. The median age of patients was 62 years (range: 42 – 75), 87% were male, all had PS 0-1and clear cell histology. Nine (67%) had IMDC intermediate risk and 6 (33%) had IMDC poor risk disease.
Overall, 36 lesions were irradiated, including 10 lung lesions (63 Gy/9 fractions), 10 bone lesions (24-40 Gy/5-8 fractions), 7 lymph nodes (30-50 Gy/6-10 fractions), 4 kidney lesions (40 Gy/5 fractions), and 5 other lesions.

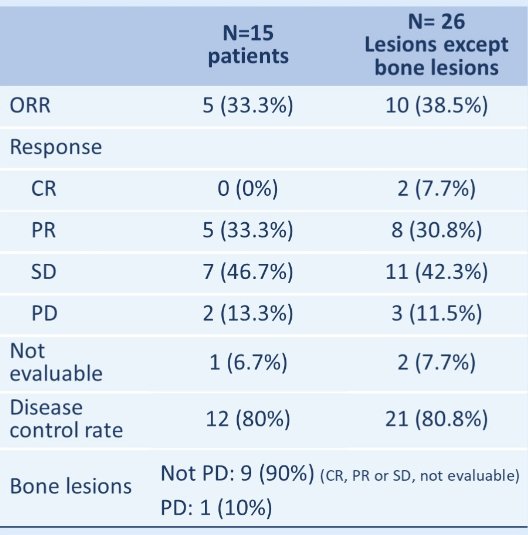
On a per-patient level, the ORR was 33.3% (CR: 0, PR: 5, SD: 7, PD: 2, not evaluable: 1). On a per-lesion level, this was 27.8% (CR: 2, PR: 8, SD: 11, PD: 3, not evaluable:12).
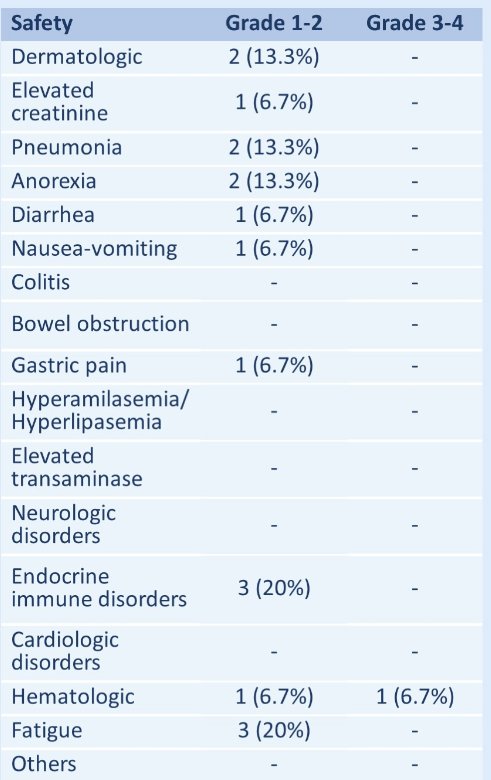
The most common treatment-related toxicities were fatigue (20%), hypothyroidism (20%), and pneumonia (13.3%), all grade 1-2. One patient suffered grade 3 treatment-related hematologic toxicity. There were no treatment-related deaths. Median PFS and OS were not reached.
The investigators concluded that the combination of all-lesion SBRT with ICI is feasible and associated with an accepted safety profile. Encouraging antitumor activity was observed warranting further investigations. Clinical trial information: ChiECRCT20210046.
Presented by: Jing Liu, MD, PhD, Shandong Cancer Hospital and Institute, Jinan, China
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 Genitourinary (GU) American Society of Clinical Oncology (ASCO) Annual Meeting, San Francisco, Thurs, Feb 16 – Sat, Feb 18, 2023.


