(UroToday.com) The 2023 GU ASCO annual meeting included an oral abstract session on renal cell carcinoma (RCC), featuring a presentation by Dr. Brian Shuch discussing results from ZIRCON, a phase 3 study of 89Zr-DFO-girentuximab for PET/CT imaging of clear cell RCC. The increasing detection of renal masses presents a significant patient management challenge. Diagnostic options include cross-sectional imaging, which cannot reliably differentiate benign and malignant renal masses, and biopsy, which is invasive and subject to sampling errors. Furthermore, 20-30% of resected small renal masses are benign, and clear cell RCC is 75% of RCC and causes ~90% of deaths. These limitations highlight the unmet need for accurate noninvasive method for pre-treatment risk stratification, similar to the recent advancements in PSMA imaging in prostate cancer. Girentuximab is a monoclonal antibody that targets carbonic anhydrase IX, an enzyme highly expressed in clear cell renal carcinoma. Radiolabeled 89Zr-DFO-girentuximab (TLX250-CDx) is highly specific for carbonic anhydrase IX and can aid differentiation between clear cell RCCs and other renal lesions:
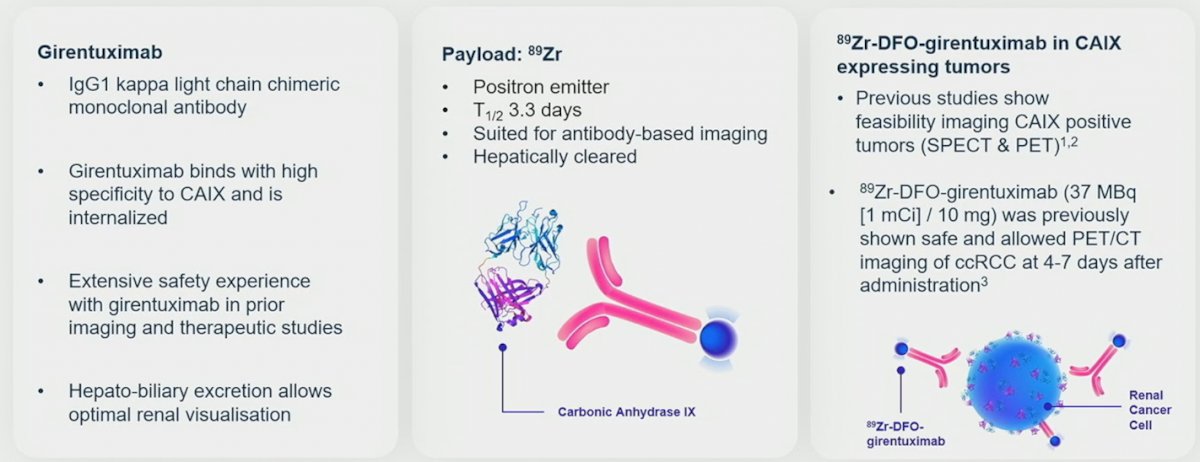
The ZIRCON study evaluated the performance of TLX250-CDx PET/CT for the detection of clear cell RCC in adult patients with indeterminate renal masses.
ZIRCON was an open-label, multicenter clinical trial. Patients with indeterminate renal masses (≤ 7 cm; tumor stage cT1) who were scheduled for partial nephrectomy within 90 days from planned TLX250-CDx administration were eligible. Enrolled patients received a single dose of TLX250-CDx IV (37 MBq ± 10%; 10 mg girentuximab) on Day 0 and underwent PET/CT imaging on Day 5 (± 2 days) prior to surgery:
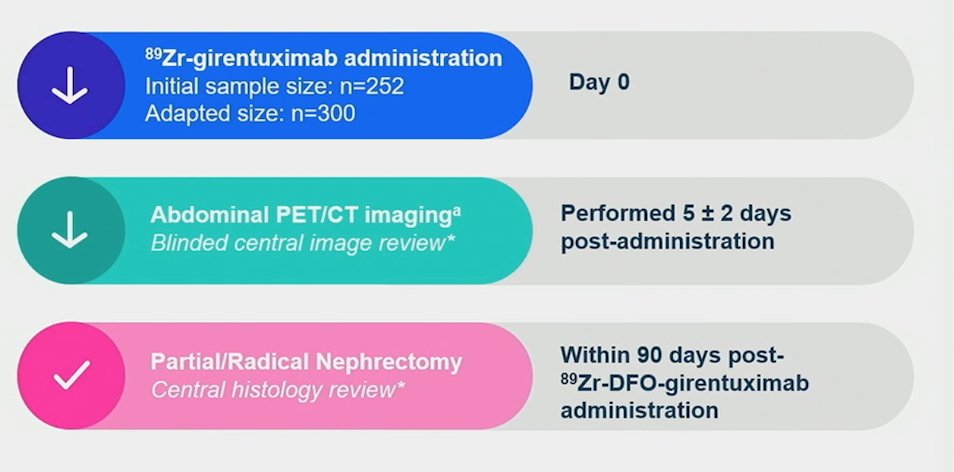
Blinded central histology review determined clear cell RCC status. The co-primary objectives were to evaluate both the sensitivity and specificity of TLX250-CDx PET/CT imaging in detecting clear cell RCC in patients with indeterminate renal masses, using histology as the standard of truth. Key secondary objectives included sensitivity and specificity of TLX250-CDx PET/CT imaging in the subgroup of patients with indeterminate renal masses ≤ 4 cm (cT1a). Other secondary objectives included positive and negative predictive values, safety, and tolerability. The Wilson 95% confidence intervals (CI) lower bound for sensitivity and specificity had to be > 70% and 68% respectively for ≥ 2 independent readers to declare the study successful.
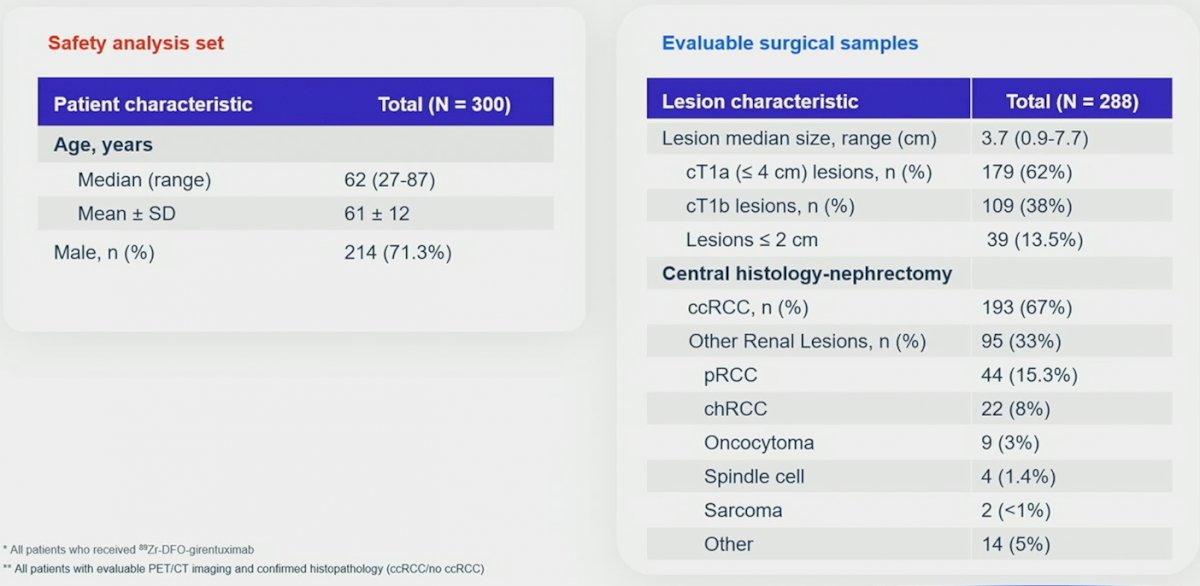
There were 300 patients enrolled between August 2019 and August 2022 among 36 sites in 9 countries that received TLX250-CDx:

The median age was 62 years (range: 27-87), and 71.3% of patients were male. Of 288 patients with central histopathology of surgical samples, 193 (67%) had clear cell RCC, and 179 (62%) had cT1a disease:
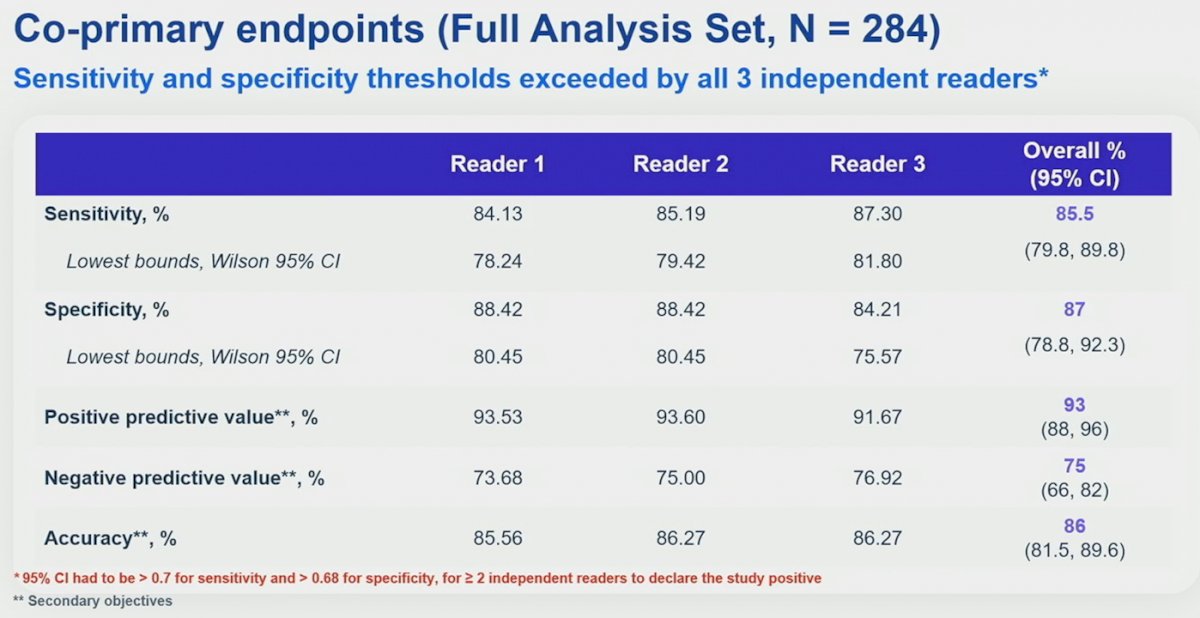
Of 284 evaluable patients included in primary analysis, the average across all 3 readers for sensitivity and specificity was 86% [95% CI 80%, 90%] and 87% [95% CI 79%, 92%], respectively, for co-primary endpoints, and 85% [95% CI 77%, 91%] and 90% [95% CI 79%, 95%], respectively, for key secondary endpoints. For all readers, the lower boundaries of 95% CI for co-primary and key secondary endpoints were > 75%. For all evaluable patients, positive and negative predictive values were ≥ 91.7% and ≥ 73.7%, respectively:
Very few adverse events were considered possible or related to 89Zr-DFO-girentuximab. Most AEs were mild, with only 18 patients (6%) having a Grade >= 3 treatment emergent adverse event. The adverse event pattern was consistent with post-surgical complications related to the nephrectomy, and no unexpected safety signals were observed. Dr. Shuch then discussed two clinical cases, first a patient with 3.5 cm complex cyst that was 89Zr-DFO-girentuximab positive and who ultimately underwent surgical intervention:

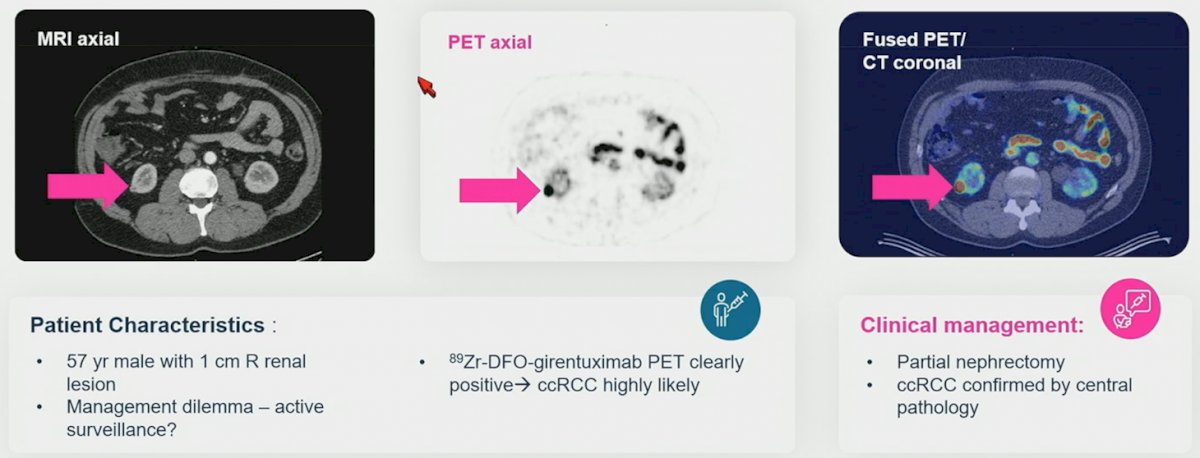
The second case was a patient with a 1 cm renal mass who was adamant about having surgery, and subsequently had a positive 89Zr-DFO-girentuximab scan:
Dr. Shuch concluded his presentation by discussing results from ZIRCON, a phase 3 study of 89Zr-DFO-girentuximab for PET/CT imaging of clear cell RCC with the following summary messages:
- The ZIRCON phase 3 pivotal study with 89Zr-DFO-girentuximab met its primary endpoint exceeding the sensitivity and specificity thresholds
- Key secondary endpoints were met, demonstrating similar performance in small masses (cT1), exceeding the sensitivity and specificity thresholds
- The favorable safety and tolerability profile of 89Zr-DFO-girentuximab was confirmed
Dr. Shuch’s take home points are as follows:
- 89Zr-DFO-girentuximab improves the identification of clear cell RCC compared to conventional cross-sectional metrics
- 89Zr-DFO-girentuximab has the potential to improve care, as this “molecular biopsy” may select appropriate patients for treatment or suggest when further imaging, biopsy, or surveillance could be considered
- Girentuximab holds promise to improve clear cell RCC staging and treatment (177Lu-girentuximab), as well as image other solid tumor types
Clinical trial information: NCT03849118
Presented by: Brian M. Shuch, MD, Institute of Urologic Oncology, David Geffen School of Medicine at UCLA, Los Angeles, CA
Co-Authors: Allan J. Pantuck, Jean-Christophe Bernhard, Michael A. Morris, Viraj A. Master, Andrew Mark Scott, Charles Van Praet, Clément Bailly, Tamer Aksoy, Robin Merkx, David M. Schuster, Sze Ting Lee, Neeta Pandit-Taskar, Alice C. Fan, Libuse Tauchmanova, Phillip Allman, Kavita Vadali, Colin Hayward, Peter Mulders
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2023 Genitourinary (GU) American Society of Clinical Oncology (ASCO) Annual Meeting, San Francisco, Thurs, Feb 16 – Sat, Feb 18, 2023.


