(UroToday.com) The 2023 GU ASCO annual meeting included an oral abstract session on renal cell carcinoma (RCC), featuring a presentation by Dr. Mauricio Burotto discussing 3-year follow-up from the phase 3 CheckMate 9ER trial assessing nivolumab plus cabozantinib vs sunitinib for first-line treatment of advanced RCC. First-line nivolumab plus cabozantinib demonstrated superiority over sunitinib with 25.4 months minimum follow-up (median, 32.9 months) in patients with advanced RCC in the CheckMate 9ER trial.1 At the 2023 GU ASCO annual meeting, Dr. Burotto and colleagues reported survival, response per blinded independent central review, and safety after 3 year minimum follow-up in all randomized patients and by IMDC risk score.
Patients were randomized 1:1 (stratified by IMDC risk score, tumor PD-L1 expression, and region) to nivolumab 240 mg flat dose IV Q2W + cabozantinib 40 mg PO QD vs sunitinib 50 mg PO for 4 weeks (6-week cycles) until disease progression or unacceptable toxicity (maximum nivolumab treatment, 2 year):
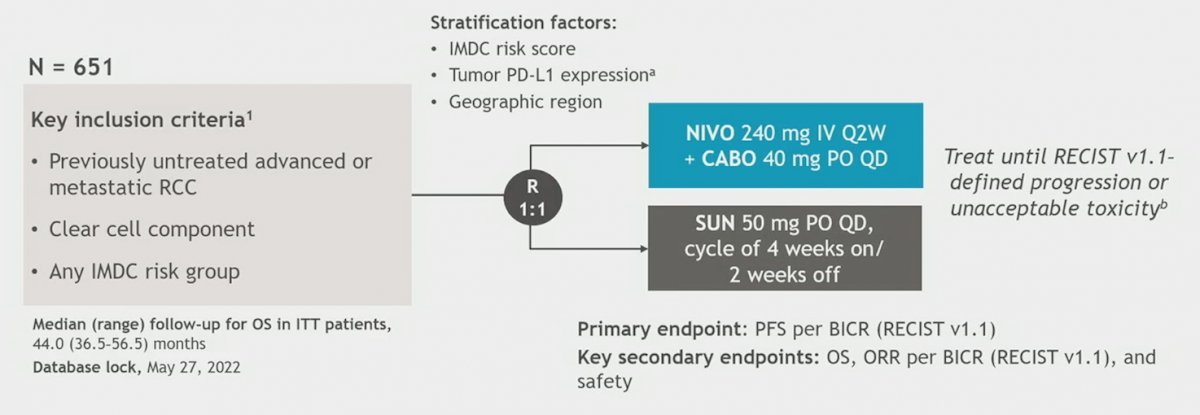
The primary endpoint was progression-free survival (PFS) by blinded independent central review. Secondary endpoints included overall survival (OS), objective response rate (ORR) by blinded independent central review, and safety.
In total, 323 patients were randomized to nivolumab plus cabozantinib and 328 to sunitinib. With 36.5 months minimum follow-up (median, 44.0 months), PFS and OS benefits were maintained with nivolumab plus cabozantinib vs sunitinib in intent-to-treat patients. Median PFS was 16.6 vs 8.4 months (HR 0.58, 95% CI 0.48–0.71):

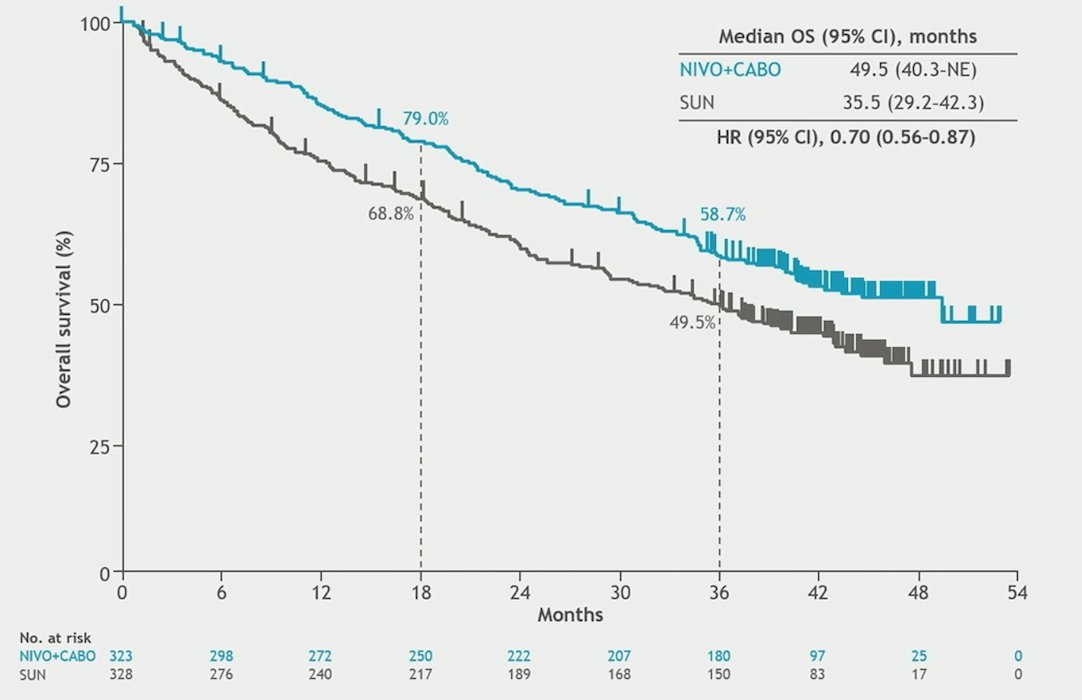
Median OS was 49.5 vs 35.5 months (HR 0.70, 95% CI 0.56–0.87):
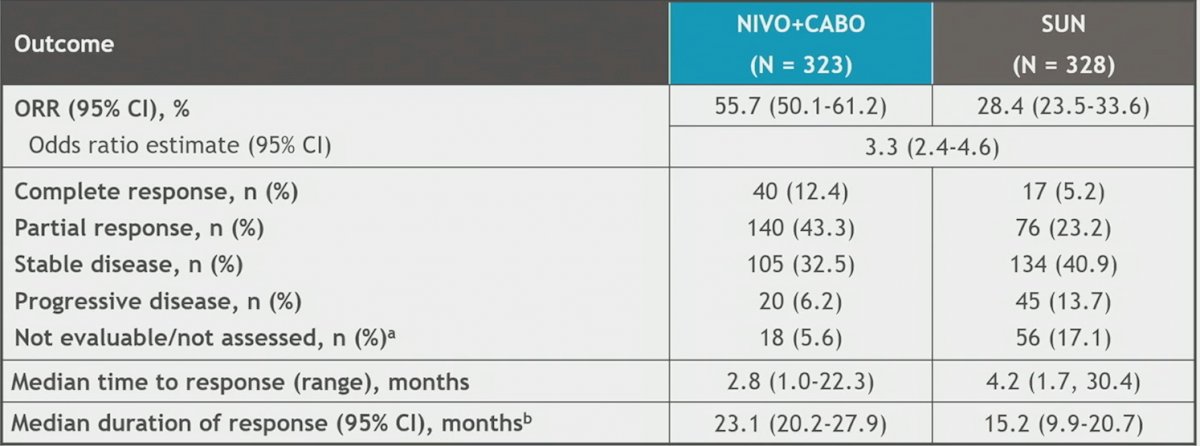
ORR was higher with nivolumab plus cabozantinib vs sunitinib (55.7% [95% CI 50–61] vs 28.4% [95% CI 24–34]), and 12.4% vs 5.2% of patients achieved complete response, respectively. Median duration of response was 23.1 vs 15.2 months for nivolumab plus cabozantinib vs sunitinib:
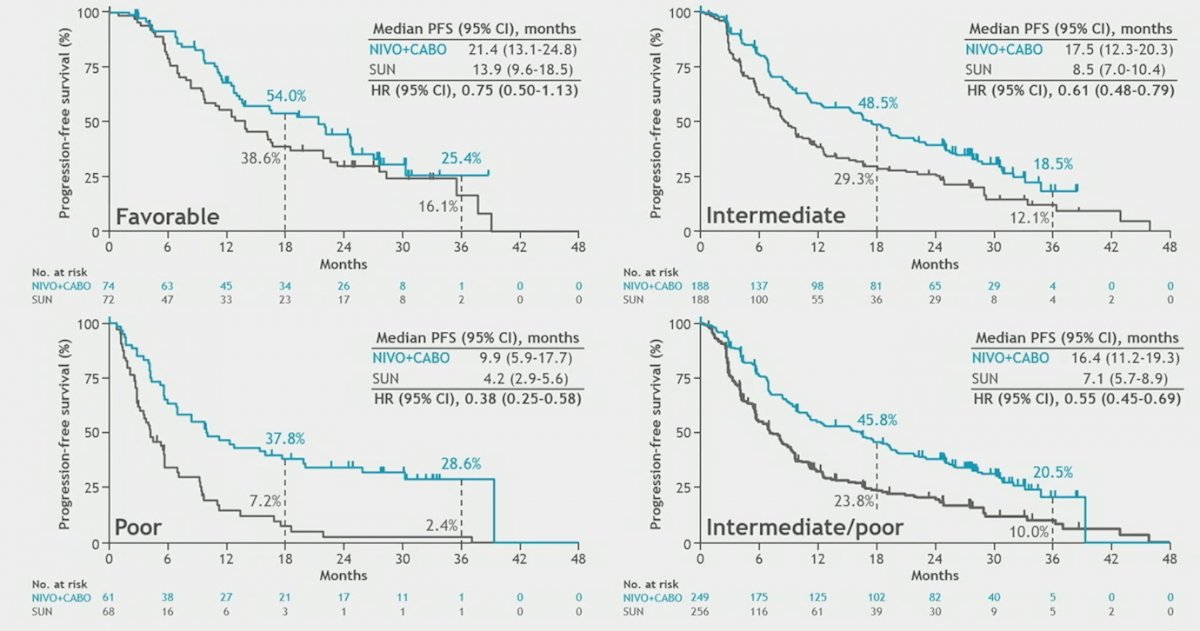
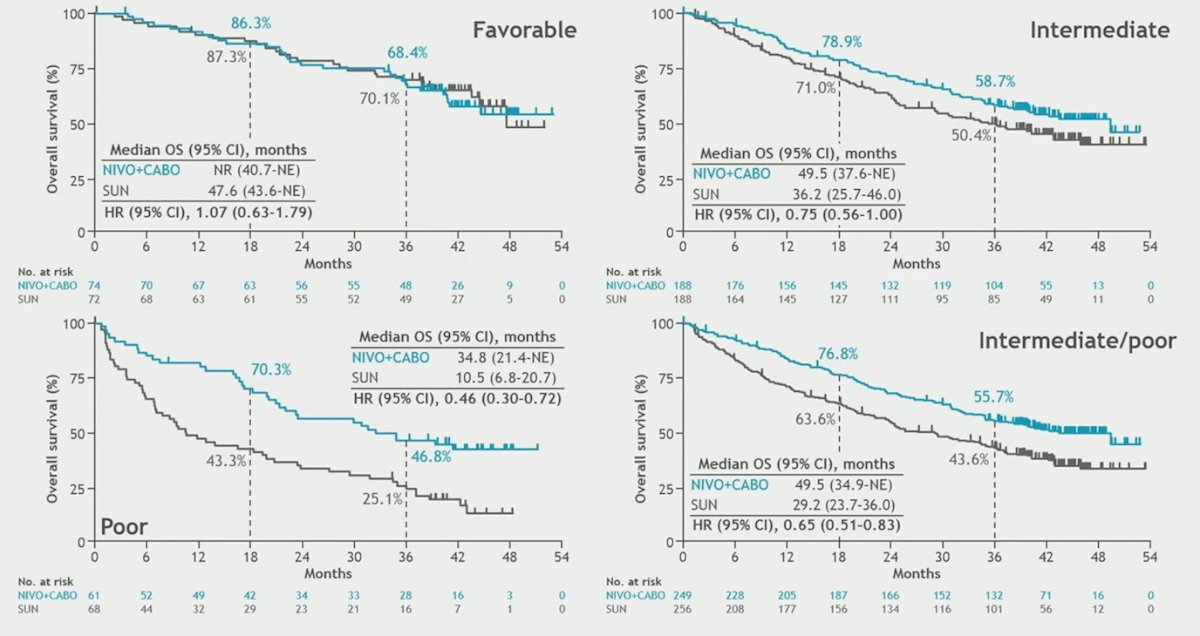
PFS by blinded independent central review by IMDC risk group showed that the combination of nivolumab plus cabozantinib was significantly beneficial in all risk groups, except for IMDC favorable risk:
Additionally, the combination of nivolumab plus cabozantinib showed OS benefit in IMDC intermediate/poor and poor risk patients:
Any-grade treatment-related adverse events occurred in 97% vs 93% of patients treated with nivolumab plus cabozantinib vs sunitinib (grade ≥ 3 treatment-related adverse events, 67% vs 55%). Treatment-related adverse events led to discontinuation of cabozantinib only in 10% of patients, nivolumab only in 10% of patients, nivolumab plus cabozantinib in 7% of patients, nivolumab or cabozantinib in 28% of patients, and sunitinib in 11% of patients.
Finally, time to subsequent therapy for patients who completed 2 years of nivolumab was 20.6 months (95% CI 7.9, not reached):

Dr. Burotto concluded his presentation discussing 3-year follow-up from the phase 3 CheckMate 9ER trial with the following concluding messages:
- After 3 years of minimum follow-up, survival and response benefits were maintained with nivolumab plus cabozantinib and remained consistent with previous follow-ups
- Median OS with nivolumab plus cabozantinib improved by 11.8 months since the previous data cut
- Responses with nivolumab plus cabozantinib were durable, with higher complete response rates with nivolumab plus cabozantinib vs sunitinib regardless of IMDC risk group
- No new safety signals emerged with additional follow-up in either arm
- These results continue to support nivolumab plus cabozantinib as a first-line treatment for patients with advanced RCC
Presented by: Mauricio Burotto, Bradford Hill Clinical Research Center, Santiago, Chile
Co-Authors: Thomas Powles, Bernard Escudier, Andrea B. Apolo, Maria Teresa Bourlon, Amishi Yogesh Shah, Cristina Suárez, Camillo Porta, Carlos H. Barrios, Martin Richardet, Howard Gurney, Elizabeth R Kessler, Yoshihiko Tomita, Jens Bedke, Saby George, Christian Scheffold, Peter Wang, Viktor Fedorov, Robert J. Motzer, Toni K. Choueiri
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2023 Genitourinary (GU) American Society of Clinical Oncology (ASCO) Annual Meeting, San Francisco, Thurs, Feb 16 – Sat, Feb 18, 2023.
References:
- Choueiri TK, Powles T, Burotto M, et al. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2021 Mar 4;384(9):829-841.


