(UroToday.com) The 2023 GU ASCO annual meeting included a session on prostate cancer, featuring a presentation by Dr. Frederic Pouliot discussing a secondary analysis of results from the CONDOR study, specifically changes in planned disease management after piflufolastat F18 PET/CT in men with biochemically recurrent prostate cancer and low PSA levels. Piflufolastat F18 is a PSMA-targeted radiopharmaceutical approved in the US for imaging prostate cancer patients both at the time of initial staging and at disease recurrence. In the phase 3 CONDOR trial of patients with biochemically recurrent prostate cancer, Dr. Pouliot and colleagues previously reported that nearly two-thirds (63.9%; 131/205) of participants had a change in their intended disease management plan based on pre- and post-piflufolastat F18 PET/CT management questionnaires completed by the treating physicians.1 The clinical utility of piflufolastat F18 scanning in men with very low/low PSA levels (<0.5 ng/mL) and a detection rate of ~36% has not been previously described. At the GU ASCO 2023 annual meeting, Dr. Pouliot and colleagues report the changes in intended management in this subset of patients.
Men ≥18 years of age with a rising PSA after definitive prostate cancer therapy and negative or equivocal imaging were enrolled. A single ~9 mCi (333 MBq) dose of piflufolastat F18 was administered followed by PET/CT from mid-thigh through skull vertex 1-2 hours later. Prior to scanning, the treating physicians completed a pre-PET management questionnaire to document the initial intended management plan for their patients based on available clinical information including baseline conventional imaging results. After PET, they completed a post-PET management questionnaire and recorded the management plan in light of PET findings. Treatment recommendations that differed from the pre-scan recommendations were reported as changes in the intended management plan.
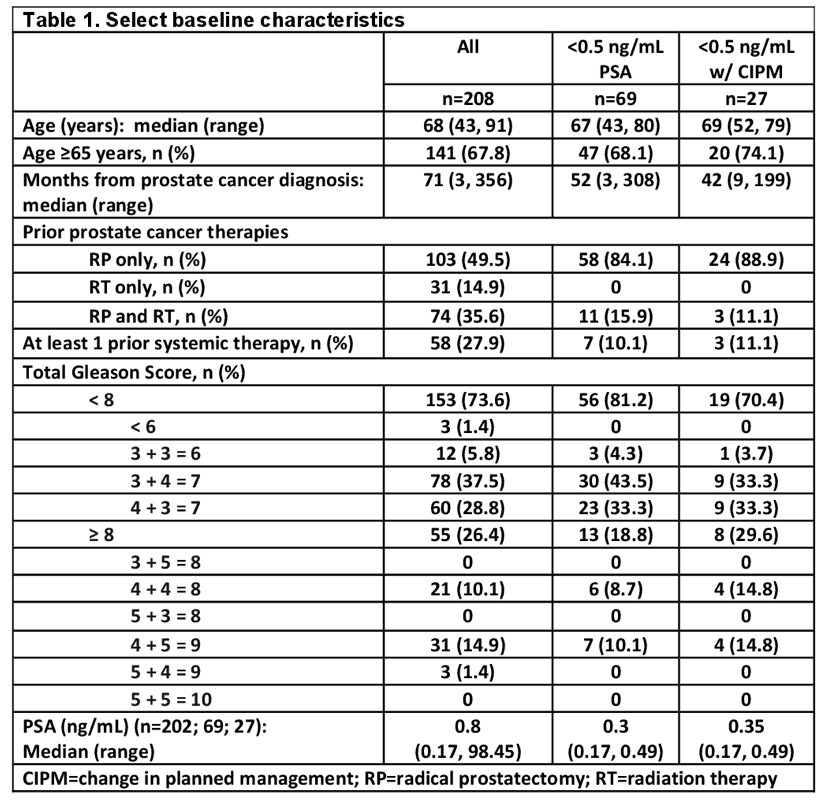
Overall, there were 208 men (median PSA 0.8 ng/mL [range 0.17-98.45], n=202) that underwent piflufolastat F18-PET/CT. Secondly, there were 200 evaluable patients that had both a baseline PSA value and completed management questionnaires. As follows are select baseline characteristics:
Of 131 patients with a recorded change in intended management, 127 had an evaluable baseline PSA level. Of the 69 patients with baseline PSA levels ≤0.5 ng/mL, 27 (39.1%) recorded a change in intended disease management based on positive (n=20) or negative (n=7) PET/CT. A summary of the change in planned medical management is as follows:

This included salvage local to systemic therapy (n=15), systemic to local therapy (n=3), observation to treatment (n=5), and treatment to observation (n=4). An additional 15 patients (21.7%) had recommended bidirectional change in management (e.g., salvage radiotherapy + ADT) and are excluded in this report. Intensification of intended treatment (salvage local therapy to systemic therapy; observation to initiating therapy) occurred in 74.1% (20/27) of patients. In 18 of 20 patients that were intensified had a positive piflufolastat lesion. 12 of the 15 with a change from salvage local to systemic therapy had ADT added to radiotherapy, while the three remaining patients were changed to systemic ADT alone. An example of a patient with an intensified treatment plan is as follows:
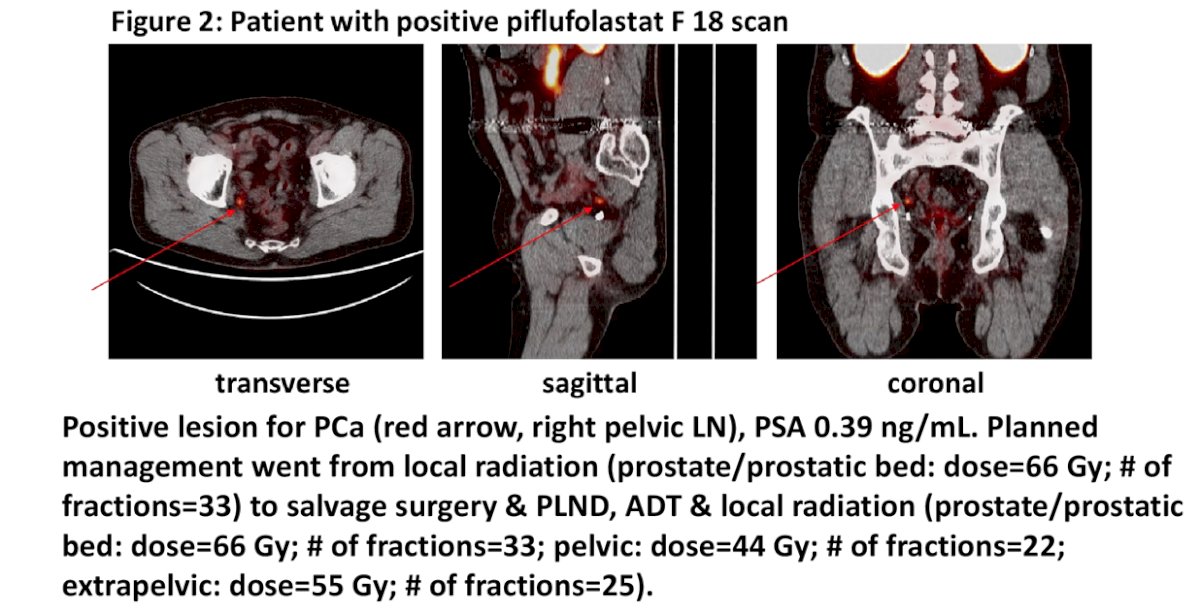
Dr. Pouliot concluded his presentation discussing a secondary analysis of results from the CONDOR study, specifically changes in planned disease management after piflufolastat F18 PET/CT in men with biochemically recurrent prostate cancer and low PSA levels with the following concluding messages:
- The frequency of changes in intended disease management observed in biochemically recurrent prostate cancer patients with low baseline PSA levels (≤0.5 ng/mL) was 39.1%
- Both negative and positive PET/CT results impacted treatment recommendations and can provide useful and actionable information
- This analysis supports the clinical utility of piflufolastat F18 PET/CT in men with low PSA levels (0.2 ng/mL – 0.5 ng/mL)
Presented by: Frederic Pouliot, MD, PhD, FRCSC, Cancer Research Center, Centre Hospitalier Universitaire (CHU) de Québec-Université Laval, Laval, Quebec, Canada
Co-Authors: Michael A. Gorin, Steven P. Rowe, Lawrence Saperstein, Bela Stephen Denes, Vincent A. DiPippo, Nancy Stambler, Michael J. Morris, Barry A. Siegel
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2023 Genitourinary (GU) American Society of Clinical Oncology (ASCO) Annual Meeting, San Francisco, Thurs, Feb 16 – Sat, Feb 18, 2023.
References:
- Morris MJ, Rowe SP, Gorin MA, et al. Diagnostic Performance of 18F-DCFPyL-PET/CT in Men with Biochemically Recurrent Prostate Cancer: Results from the CONDOR Phase III, Multicenter Study. Clin Cancer Res. 2021 Jul 1;27(13):3674-3682.


