(Urotoday.com) On the first day of the American Society for Clinical Oncology (ASCO) Genitourinary Cancer Symposium 2023 focussing on prostate cancer, Dr. Manish Kohli presented in Poster Session A on a cost-effectiveness analysis comparing seven different treatment approaches for patients with metastatic hormone-sensitive prostate cancer (mHSPC).
Over the past few years, treatment intensification employing either docetaxel or a novel hormonal therapy in addition to androgen deprivation therapy (ADT) has become standard of care for patients with metastatic castration-sensitive prostate cancer (mCSPC), based on consistently demonstrated survival benefits. Despite a number of available treatment options, there are no current predictive biomarkers to guide treatment selection. Thus, this paper aimed to determine the cost-effectiveness of each of the treatment approaches from the US public sector perspective with a lifetime horizon.
The authors developed a partitioned survival model in which mHSPC patients transitioned between three health states (progression free, progressive disease to castrate resistance state, and death) at monthly intervals based on Weibull survival model estimated from published Kaplan-Meier curves using a network meta-analysis. They relied upon a Bayesian network meta-analysis of seven clinical trials which included 7,208 patients. The efficacy output of the model was presented as quality-adjusted life-years (QALYs) with utility values obtained from published literature while included costs captured those associated with treatment regimens and subsequent therapies, terminal care, and for managing grade 3-4 drug related adverse events, and were obtained from the Federal Supply Schedule and published literature.

The authors found that average lifetime costs ranged from $154,139 (Abiraterone Acetate+Prednisone+ADT) to $770,848 (Darolutamide+Docetaxel+ADT) and mean QALYs ranged from 3.33 (ADT alone) to 5.08 (Enzalutamide+ADT). In comparisons between treatment approaches, all treatment strategies other than Abiraterone Acetate+Prednisone+ADT and Enzalutamide+ADT were eliminated due to dominance. Compared to Abiraterone Acetate+Prednisone+ADT, the incremental cost-effectiveness ratio for Enzalutamide+ADT was $484,943/QALY.
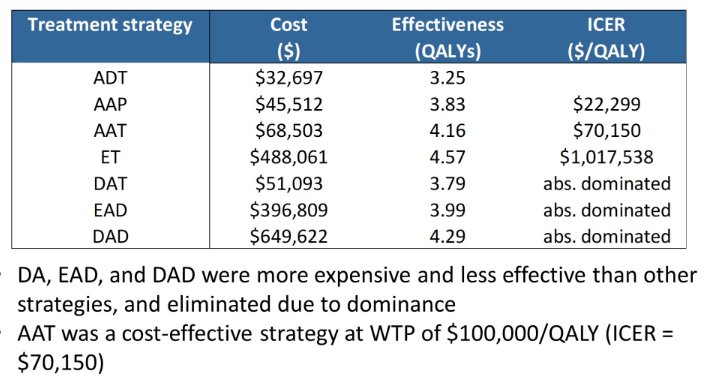
The authors therefore conclude that this simulation model among patients with mCSPC demonstrate that Enzalutamide+ADT is likely to both maximize QALYs and would the most cost-effective option with a WTP threshold as high as $500,000/QALY. However, for a WTP threshold of $100,000/QALY, Abiraterone Acetate+Prednisone+ADT was the most cost-effective treatment strategy.



