(UroToday.com) On the first day of the American Society for Clinical Oncology (ASCO) Genitourinary Cancer Symposium 2023 focussing on prostate cancer, Dr. Kamal Kant Sahu presented in Poster Session results of a phase II study assessing rucaparib monotherapy in patients with nonmetastatic, hormone-sensitive prostate cancer (nmHSPC) with a “BCRAness” genotype.
There is an extensive body of literature suggesting that patients with prostate cancer who have DNA repair defects (aka “BRCAness”) generally have more aggressive disease and a worse overall prognosis compared to men without BRCAness. Following local therapy, biochemical recurrence (BCR) is typically the earliest sign of oncologic progression and may occur in up to one-third of men with prostate cancer after treatment with local therapy. Given that tumors in patients with DNA repair defects are sensitive to the synthetic lethal effect of PARP inhibitors, the authors hypothesized that treatment with rucaparib monotherapy in men with systemic treatment naïve high-risk nmHSPC would result in acceptable disease control, allow a delay of androgen deprivation therapy (ADT) and the onset of metastasis.
To test this hypothesis, the authors performed a single-arm, open-label, phase II trial (NCT03533946). They enrolled men with histologically proven prostate adenocarcinoma who had the presence of one of the following alterations in (by soft tissue or liquid biopsy): BRCA1, BRCA2, ATM, BARD1, BRIP1, CHEK1, CHEK2, FANCA, NBN, PALB2, RAD51C, RAD51D, RAD51, RAD51B. Additionally, men had to have no radiographic evidence of metastatic disease by conventional scans and a PSA doubling time of ≤10 months. The primary endpoint of interest was PSA progression-free survival (PSA-PFS) with secondary endpoints including safety, the proportion of patients with a PSA 50% response (PSA50), and the proportion of patients with an undetectable PSA.
The authors enrolled 7 patients with the following pathogenic alterations: ATM (n=3), BRCA2 (n=2), BRCA1 (n=1), BRIP1 (n=1), RAD51(n=1). Each enrolled patient received treatment with rucaparib at 600 mg twice daily. A 4-week treatment duration comprised one cycle. Over a median duration of follow-up of 18 months, enrolled men received a median of 20 cycles (range 4-42) of rucaparib.
The median PSA-PFS was 35.37 months (95% CI: 0 - 85.11 months). In terms of oncologic endpoints, two of seven patients achieved PSA50; both of whom had nadir PSA of undetectable levels.
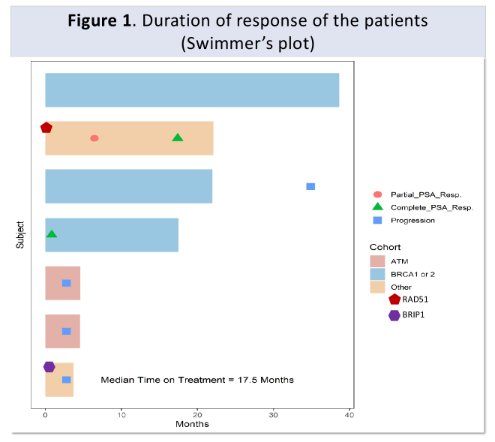
In terms of toxicity, grade 3 or greater adverse events (AEs) occurred in two patients, on each with anemia and rash. No dose-limiting toxicities or severe AEs were seen. The study was prematurely terminated in June 2022 after the accrual of 7 patients due to widespread adoption of next-generation scans (e.g., PSMA-PET) for patients with BCR.
Based on these limited data, the authors concluded that rucaparib demonstrated acceptable toxicity and preliminary efficacy in patients with biochemical recurrence following local therapy for prostate cancer. However, these data are limited due to the small cohort and lack of a comparison arm.
Presented by: Kamal Kant Sahu, MBBS, Huntsman Cancer Institute at the University of Utah

