(UroToday.com) The 2023 American Society of Clinical Oncology Genitourinary (ASCO GU) cancers symposium held in San Francisco, CA between February 16th and 18th was host to a prostate cancer poster session. Dr. Louise Emmett presented the rationale and study design for the PROPELLER, a phase I trial evaluating a novel PSMA-targeted tracer, 64Cu-SAR-bisPSMA, in intermediate- to high-risk patients planned for a radical prostatectomy.
There has been an increased utilization of prostate-specific membrane antigen (PSMA)-based positron emission tomography/computed tomography (PET/CT) scans for the pre-operative staging of patients with intermediate to high-risk patients. 68Ga-PSMA-11 is one of the most widely available and commonly used tracers in this setting, with PSMA-PET/CT scans using this tracer demonstrating improved sensitivity and specificity compared to conventional imaging.1 However, there are significant limitations to the practical use of 68Ga-PSMA-11 which prohibit widespread option in all centers. Copper-64 has a comparatively longer half-life (t1/2=12.7 hours versus 68 minutes for 68Ga-PSMA-11) and shorter positron range (mean positron range=0.56 mm versus 3.5 mm for 68Ga-PSMA-11). As such, a novel PSMA-targeting radiotracer labelled with this radionuclide, 64Cu-SAR-bisPSMA, has the potential to provide practical advantages over 68Ga-PSMA-11. From a practical standpoint, the 12.7-hour half-life of Cu-64 allows for the central manufacturing of 64Cu-SAR-bisPSMA with a product shelf-life of up to 2 days. This longer half-life enables PET imaging from 1 to 72 hours after administration, which allows for greater flexibility in terms of patients scheduling. This may also translate into detection of additional lesions due to increased Standardized Uptake Values (SUV) in the lesions relative to the background following the biological clearance of the tracer from organs over time. This shorter positron range may lead to improved scan resolution and, thus, improved detection of small lesions, including those that are in close proximity to each other, or to organs involved in tracer clearance, such as the bladder and kidneys.
Another important advantage of 64Cu-SAR-bisPSMA is the presence of two PSMA-targeting functional groups, compared to the one with 68Ga-PSMA-11. This can lead to improved tumor uptake and retention. This was proven to be the case in a preclinical study, where 64Cu-SAR-bisPSMA outperformed the monomeric 64Cu-SAR-PSMA with respect to both tumor uptake and retention.2
The PROPELLER trial, a multi-center blinded review, dose ranging phase I study included 30 patients with untreated, histopathological-proven, intermediate- to high-risk prostate cancer planned for a radical prostatectomy who underwent a pre-operative 68Ga-PSMA-11 PET/CT per institutional practice. These patients were subsequently allocated to one of three dose cohorts in a 1:1:3 fashion to receive either 100 MBq, 150 MBq, or 200 MBq of 4Cu-SAR-bisPSMA, respectively. A PET/CT was acquired three hours (+/- 1 hour) post-injection of 64Cu-SAR-bisPSMA. The primary endpoint was detection of primary prostate cancer on the 200 MBq 64Cu-SAR-bisPSMA PET/CTs, assessed by two independent, blinded, central readers. Lesion intensity was compared between 68Ga-PSMA-11 and 64Cu-SAR-bisPSMA PET/CT by reporting SUVmax, SUVmean, and tumor-to-background ratio (ratio of lesion SUVmax and background SUVmean) in up to five concordant lesions between the two tracer modalities (exploratory endpoint).

Baseline patient demographic are demonstrated in the table below. The median patient age was 64 years (range: 50 to 75). Almost half the patients had ISUP Grade Group 4 or 5 disease. The median PSA level was 10.49 ng/ml (SD:8.08).

64Cu-SAR-bisPSMA was well-tolerated with only a single related AE of grade 1 dysgeusia (metallic taste) reported in the 200 MBq cohort. The interval between 68Ga-PSMA-11 and 64Cu-SAR-bisPSMA PET/CT scans was 2-50 days (median 20.5).

For both readers, 200MBq of 64Cu-SAR-bisPSMA scored the highest in terms of image quality. In this cohort, 64Cu-SAR-bisPSMA and 68Ga-PSMA-11 were able to detect primary prostate cancer in 100% and 77.8% of patients for Reader 1 and 85.7% and 83.3% of patients for Reader 2, respectively. The rest of the scans were indeterminate, with no scan deemed negative.

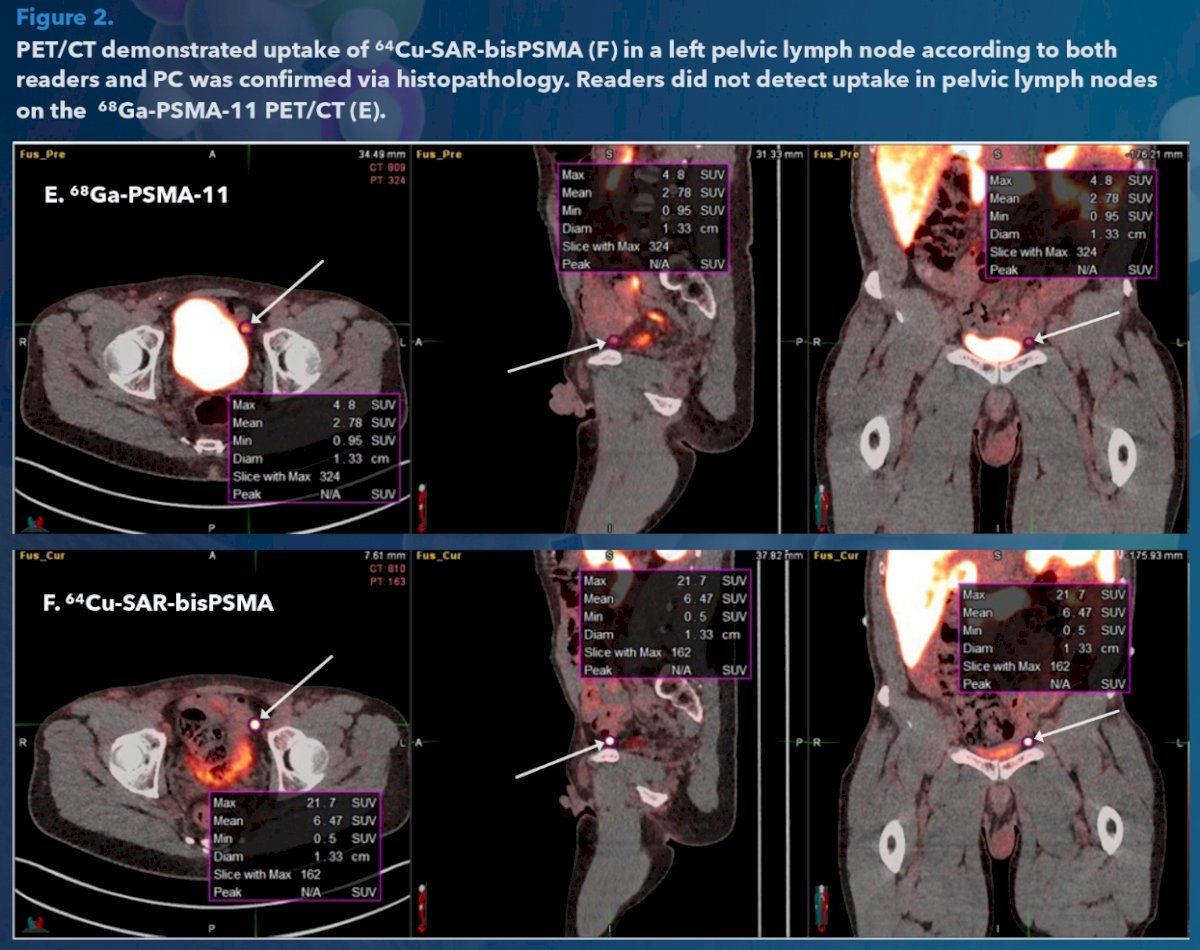
The resulting true positive rate (i.e. sensitivity) and false negative rates were similar for both tracers. Uptake of 64Cu-SAR-bisPSMA showed higher SUVmax compared to 68GA-PSMA-11. Additional secondary disease, in a pelvic lymph node, was detected on 64Cu-SAR-bisPSMA PET/CT compared to 68Ga-PSMA-11 PET/CT and verified by histopathology.

Based on these results, the authors concluded that 64Cu-SAR-bisPSMA, a new candidate for PCa imaging, is shown to be safe, well-tolerated, and efficacious for imaging PSMA-expressing lesions. A dose of 200MBq was determined as the optimal dose for future trials. Further studies to evaluate 64Cu-SAR-bisPSMA as an imaging agent in biochemical recurrence of prostate cancer are underway.
Study Research Funding:Pharmaceutical/Biotech Company, Clarity Pharmaceuticals
References:
- Hofman MS, et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study. Lancet 2020;395(10231):1208-1216.
- Zia NA, et al. A Bivalent Inhibitor of Prostate Specific Membrane Antigen Radiolabeled with Copper-64 with High Tumor Uptake and Retention. Angew Chem Int Ed Engl 2019;58(42):14991-14994.
Presented by: Louise Emmett, MD, MBChB, FRACP, Professor and Director of Theranostics and Nuclear Medicine, St Vincent's Hospital, Sydney, Australia
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, San Francisco, CA, February 16th – February 18th, 2023


