(UroToday.com) The 2023 American Society of Clinical Oncology Genitourinary (ASCO GU) cancers symposium held in San Francisco, CA between February 16th and 18th was host to a prostate cancer poster session. Dr. Arjun Gupta presented the results of an ad-hoc analysis of the E3805 CHAARTED trial evaluating the effect of cerebral dopamine metabolism genetic polymorphism on patient-reported quality-of-life.
Specific genetic variants in neurotransmitter metabolism have been associated with quality-of-life outcomes. One such variant is the rs4680 SNP of Carboxy-O-Methyltransferase (COMT), which is associated with reduced symptoms in placebo studies. This variant is corelated with reduced COMT enzymatic activity, higher cerebral dopamine levels, and improved mood levels. The association/interaction between this germline variant and cancer-directed treatment on quality-of-life outcome remains unexplored. As such, the authors conducted an ad-hoc analysis of the E3805 CHAARTED trial, with an a priori hypothesis of improved pain, quality-of-life, and patient-reported outcomes among patients with COMT rs4680 SNP variants, compared to those with the wildtype (WT) COMT variant.
E3805 CHAARTED was a prospective phase III trial that compared the efficacy and safety of early treatment intensification with docetaxel addition to ADT in patients with metastatic hormone sensitive prostate cancer.1 Patient reported outcomes (PROs) were collected at baseline, three, six, nine, and 12 months. Genotyped blood samples prior to treatment were available. Patient-reported outcomes were compared between patients with COMT WT versus rs4680 SNP, within treatment arms longitudinally. Patient reported outcomes were evaluated using the Brief Pain Inventory (BPI) score classified as: no (0), minimal (1), or more (≥2) pain, and BPI interference (range 0-10, 0= no pain or interference), and the Functional Assessment of Cancer Therapy-Prostate (FACT-P, higher score= better QOL, clinically important difference (CID)= 6). Quality of life outcomes were summarized/characterized over time using descriptive statistics. The associations between SNP and quality of life outcomes in each arm were compared using the Fisher’s exact test, Wilcoxon rank sum test, and mixed effects modeling.
Of 790 evaluable patients., 550 (70%) had evaluable SNP data. In the trial intervention arm of docetaxel + ADT, SNPs were not associated with patient-reported outcomes at any time point. In contrast, among patients receiving ADT alone, when compared to WT, rs4680 was associated with:
- Less pain at 3 months
- Less interference at 3, 6, and 9 months
- Better quality of life at 6 months (met criteria for clinically important difference).
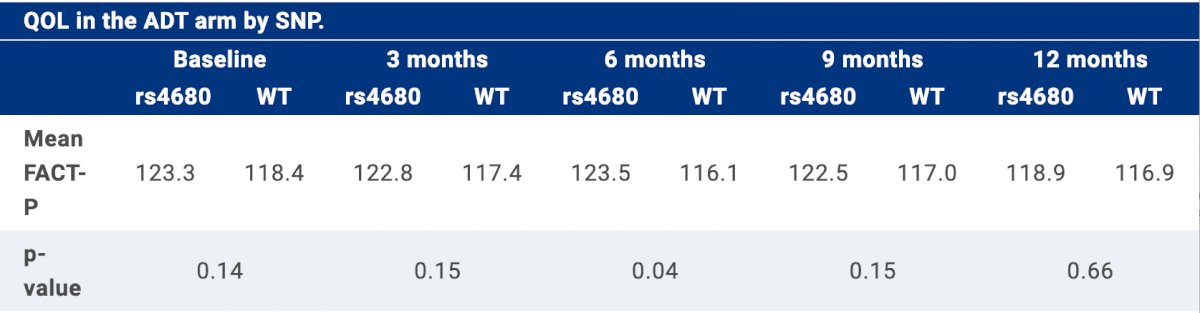

The authors concluded that rs4680 SNP is associated with lower pain levels and superior quality of life among patients with mHSPC treated with ADT, but not chemohormonal therapy. To date, this is the first hypothesis-driven genotyping study demonstrating that genetics are associated with quality of life in patients with cancer, especially when treatment does not cause profound symptoms. Future work will assess additional PROs from the CHAARTED trial, as well as investigate this COMT SNP in additional treatment settings in patients with prostate cancer. If the association between the rs4680 SNP of COMT and patient-reported QoL is validated, incorporation of COMT SNP type into QoL outcomes data may enhance clinical decision making through more accurate expectations of QoL outcomes.
Presented by: Arjun Gupta, MD, Assistant Professor of Medicine, University of Minnesota, Minneapolis, Minnesota
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, San Francisco, CA, February 16th – February 18th, 2023.
References:
- Sweeney CJ, et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N Engl J Med 2015;373:737-746.


