(UroToday.com) The 2023 GU ASCO annual meeting included a session on prostate cancer, featuring a presentation by Dr. Miles Hsu discussing the implementation of 18F-DCFPyL PET in clinical practice, specifically prescribing patterns and utilization at a large academic center. 18F-DCFPyL imaging was recently approved for initial staging in patients with prostate cancer and suspected metastasis and for those with biochemical recurrence. 18F-DCFPyL recently became available at the Abramson Cancer Center NCI-Designated Comprehensive Cancer Center comprising six teaching hospitals and two outpatient facilities. As real-world data on 18F-DCFPyL usage in the US are lacking, the objective of this study by Dr. Hsu and colleagues was to describe prescription patterns and utilization for 18F-DCFPyL.
This study identified 18F-DCFPyL scans performed at the Abramson Cancer Center from January 1, 2022, through May 10, 2022, and abstracted corresponding clinical data through electronic medical record review. Demographic, laboratory, pathological and clinical data and provider-level usage were characterized by review of the electronic medical record and, if necessary and feasible, a provider interview.
There were 164 18F-DCFPyL scans performed across the study period for 164 unique patients (median age 70 years, 73.8% White, 20.1% Black, 3.1% Asian, 1.8% Hispanic). Ordering providers belonged to medical oncology (54.3%), radiation oncology (42.7%), urology (2.4%), and nuclear medicine (1%). The majority of scans were ordered for isolated PSA rise (n=93, 56.7%) and initial staging (n=26, 15.9%), in concordance with FDA-approved indications:
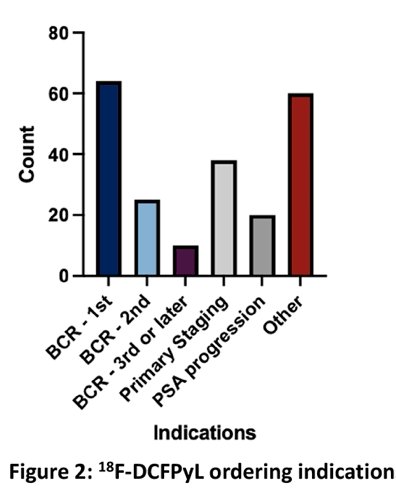
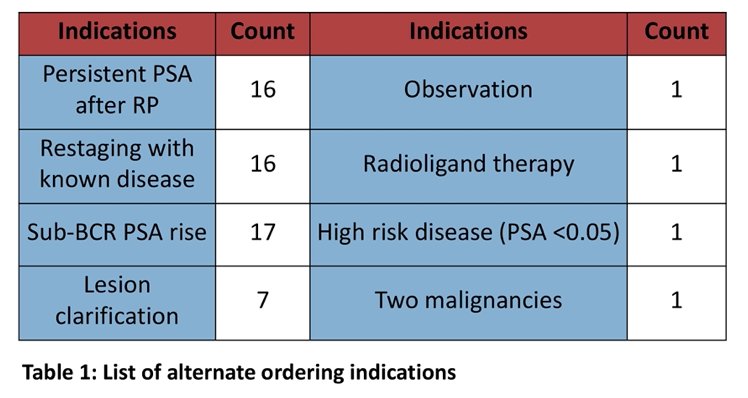
Nevertheless, many scans were ordered for alternate indications. Restaging of patients with known refractory or metastatic disease (11.0%), PSA persistence after definitive therapy (8.5%), and PSA rises below the threshold of biochemical recurrence (4.9%) were common alternate rationales for imaging. A minority of scans had a secondary indication such as radiotherapy field planning, distinguishing between two primary cancers, PSA persistence in high-risk disease, possible radioisotope therapy, and, most frequently, clarification of lesions seen on conventional imaging (n = 7):
There were 22 patients on ADT (n=17) or anti-androgens (n=11) when receiving PSMA imaging, with 5 patients on both, with a median testosterone at the time of imaging was <20 ng/dL:

Indeterminate lesions, defined as tracer avid with uncertain malignant status, were most often found in lymph nodes (n = 22) and focal rib lesions (n = 17), of which 12 were solitary, 3 were multifocal and 2 were superimposed on fractures.
Dr. Hsu concluded his presentation discussing implementation of 18F-DCFPyL PET in clinical practice with the following concluding messages:
- Shortly after adoption at a large cancer center, 18F-DCFPyL has been prescribed in various clinical settings outside of FDA approvals
- Around 1/8 were performed with patients on ADT/anti-androgens, with testosterone at castrate levels. Findings of uncertain malignant etiology included solitary (7%) and multifocal (2%) rib lesions
- These data reveal substantial variability in usage and areas of uncertainty in the interpretation of 18F-DCFPyL imaging in real-world clinical settings, underlining the need for further studies on its potential usefulness in expanded indications
Presented by: Miles Hsu, Abramson Cancer Center, Penn Medicine, Philadelphia, PA
Co-Authors: Xinhe Shan, Samuel U Takvorian, Austin Pantel, Vivek Narayan, Jeffrey Shevach, Neha Vapiwala, David J. Vaughn, Naomi B. Haas
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2023 Genitourinary (GU) American Society of Clinical Oncology (ASCO) Annual Meeting, San Francisco, Thurs, Feb 16 – Sat, Feb 18, 2023.


