(UroToday.com) Dr. Elena Castro provided a terrific discussion of PARP inhibitors in metastatic castration resistant prostate cancer (mCRPC) immediately following the presentation of updated data from TRITON3 and TALAPRO-2. She initially presented the history of PARPi in mCRPC prior to the aforementioned studies as follows.
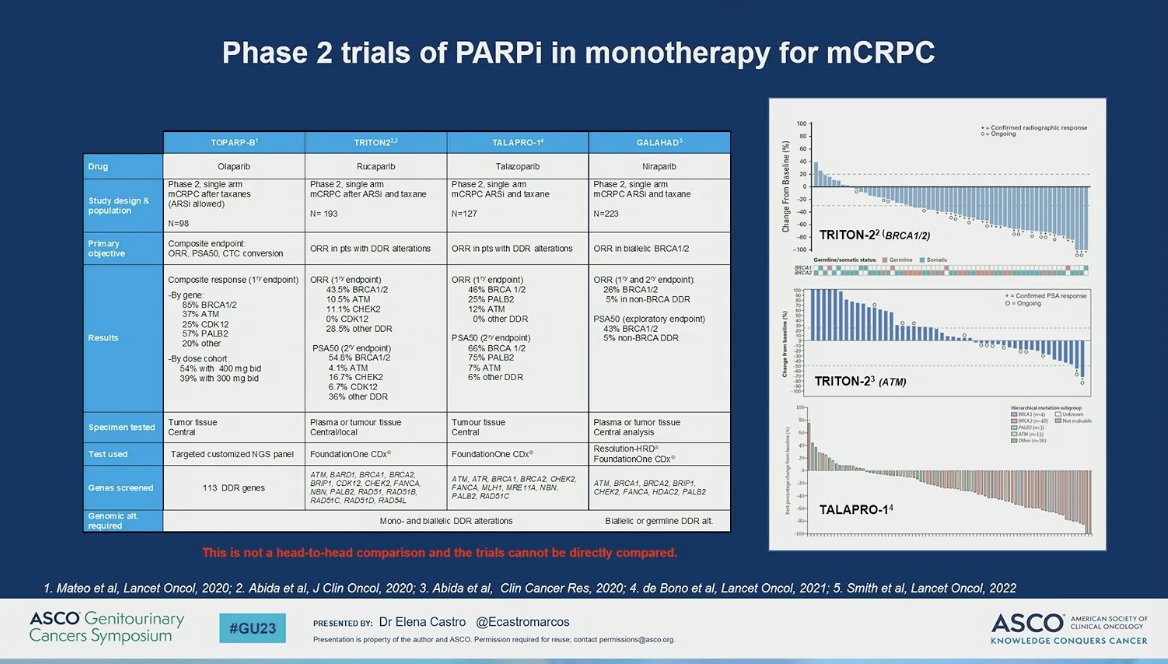
She highlighted the importance of PROfound as a biomarker-selected study in mCRPC and that benefit was demonstrated in those patients with BRCA1/2 and ATM alterations, as compared to second AR signaling inhibitor. Of note, patients were not pre-selected for these alterations but known to have them. TRITON-3 was similar in its focus on these gene alterations but different in that they were less heavily pre-treated. She highlights that the improvement in rPFS in the absence of convincing OS data as yet, as well as the absence of clear benefit in ATM-mutated patients. These data raise several questions, as proposed by Dr. Castro, including (1) do ATM-mutated patients benefit? And (2) should PARPi precede or follow docetaxel therapy. She suggests that ATM mutation does not confer a benefit to PARPi-treated patients and perhaps PARPi may be better before docetaxel when comparing to PROfound data. Because of the crossover rate, we may be able to see the directionality of docetaxel followed by PARPi and the other way around. She emphasizes also that the clear benefit of PARPi in BRCA1/2-mutated patients is also associated with their worse underlying outcome and biology. In this context, she emphasizes, as did Dr. Bryce, the important comparison of PARPi (rucaparib) versus docetaxel.
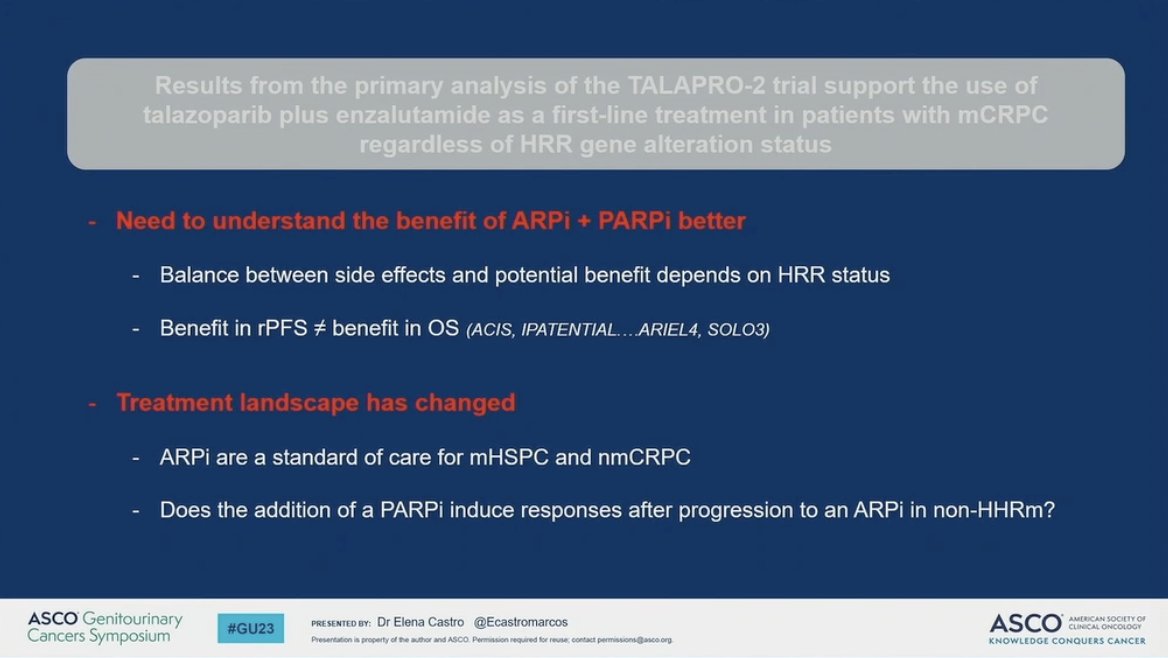
Following this, focus turned to TALAPRO-2 which demonstrated a clear benefit in rPFS for first line mCRPC with 37% reduced risk of progression or death. Subgroup analyses shown by Dr. Agarwal were slightly revised by Dr. Castro to emphasize that HRR status does indeed matter. When restricted to the analysis of “true negatives,” although exploratory and unstratified, support the synergistic effect of PARPi and ARPIs.
Dr. Castro emphasized in her presentation and during the question and answer period that management of a patient on ARPI and a PARPi are not the same. The overall hematologic-predominance in TEAEs impact patient management. To wit, patients on PARPi, almost more like those on cytotoxic chemotherapy, need closer follow up for clinical evaluation, consideration of dose adjustment, and possible transfusions.
As the end of the talk approached, Dr. Castro a table of phase III PARP studies – not for cross trial comparisons – but for orientation of the landscape of combination studies of ARPI and PARPi in mCRPC. The audience was encouraged to maintain in mind that there are relevant differences across these studies with regard to the proportion of patients with HRR, which AR axis drug is used (e.g., AR inhibitor and CYP inhibitor), and the PARP inhibitor mechanism and behavior.
Finally, Dr. Castro offered support for the work both completed and ongoing and emphasized a few key points. These include that the balance of potential benefits and side effects will be impacted by refined understanding of how ARPI and PARPi behave together. Secondly, that rPFS should be interpreted with caution with regard to expectations of OS extension. Finally, to remember that the treatment landscape has changed. The appropriateness of first line ARSI in mCRPC is compromised by earlier use of these agents, which leads to the intriguing suggestion of sensitivity rescue: following progression on ARPI, would the addition of PARPi induce responses in non-HR mutated patients?
Presented by: Elena Castro, MD, PhD, Medical Oncologist, Virgen de la Victoria University Hospital in Malaga, SpainWritten by: Jones Nauseef, MD, PhD, Assistant Professor of Medicine within the Division of Hematology and Medical Oncology, Sandra and Edward Meyer Cancer Center, and Englander Institute for Precision Medicine Weill Cornell Medicine and Assistant Attending physician at NewYork-Presbyterian Hospital. @DrJonesNauseef on Twitter during the 2023 Genitourinary (GU) American Society of Clinical Oncology (ASCO) Annual Meeting, San Francisco, Thurs, Feb 16 – Sat, Feb 18, 2023.
Related Content:
ASCO GU 2023: TALAPRO-2: Phase 3 Study of Talazoparib + Enzalutamide Versus Placebo + ENZA as First-Line Treatment in Patients with mCRPC
ASCO GU 2023: Rucaparib for Metastatic Castration-Resistant Prostate Cancer (mCRPC): TRITON3 Interim Overall Survival and Efficacy of Rucaparib vs Docetaxel or Second-Generation Androgen Pathway Inhibitor Therapy
PARP Inhibitors in Metastatic Castration Resistant Prostate Cancer - Elena Castro