(UroToday.com) Radium-223 is an FDA approved therapy for metastatic castration resistant prostate cancer (mCRPC). Its mechanism of action relies on its intrinsic radioactivity and its similarity to calcium, allowing for uptake into bones in mCRPC and delivery of alpha particles with the energy to induce double-stranded DNA breaks.
As anticipate, the effect of this drug is limited to bone metastases. Given this mechanism of action, it has been suggested that cells deficient in homologous recombination (HR), one cellular mechanism for repair of double-stranded breaks, may be more susceptible to radium-223. If true, patient selection could be refined by presence or absence of HR deficiency (HRD), yet bone metastases can be challenging for the required sequencing to determine HRD status.
Dr. David D. Yang and colleagues sought to evaluate circulating tumor DNA (ctDNA) for evidence of mutations in genes implicated in successful HR repair, thereby surmounting technical limitations of sequencing bone biopsies. They hypothesized that ctDNA would allow for broader identification of HRD in bone-predominant mCRPC and to assess association with clinical outcomes in a real-world cohort.
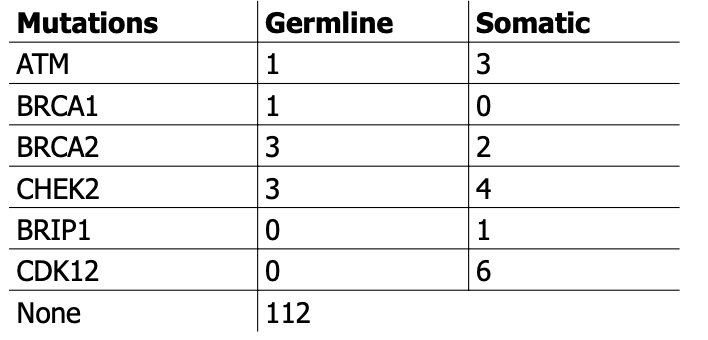
The authors identified 135 patients treated at Dana-Farber Cancer Institute between 2013 and 2021 with mCRPC who received treatment with radium-223. ctDNA was isolated from plasma collected prior to treatment and sequenced via ultra-low-pass whole genome sequencing to estimate tumor fraction using ichorCNA. Targeted sequencing via an institutional prostate cancer-specific panel of 319 genes was also performed to identify somatic and germline alterations. The HR pathway genes assessed included BRCA1, BRCA2, ATM, BARD1, BRIP1, CDK12, CHEK1, CHEK2, NBN, PALB2, RAD51, RAD51B, RAD51C, and RAD51D, and a qualifying alteration in any of these genes was consider HR deficient (HRD). Each alteration had its pathogenicity validated using ClinVar or FATHMM for germline and somatic events, respectively. The primary outcome of the study was to evaluate for association between HR deficiency status and completion of fewer than 6 cycles of radium-223, employed as a proxy for early progression, and was assessed using logistic regression.
Among the patients in the cohort, median age was 61 years (IQR, 56-67), with a majority with prior exposure to taxane (63%, n=85), and minority deemed HRD+ (17%, n=23). Tumor fraction was uniformly low (4%, IQR 3-6). 59% (n=80) receive 6 cycles of radium-223. The genes altered which qualified at HRD+ were as follows, with one patient harboring a germline and somatic alteration:
Presence of HR deficiency, as defined above, was associated with receiving fewer than 6 cycles of radium-223 (adjusted odds ratio: 0.16, [95% CI 0.05-0.48], p=0.001). Higher pre-treatment detected tumor fraction (TF) was also associated with receiving fewer that the planned 6 cycles.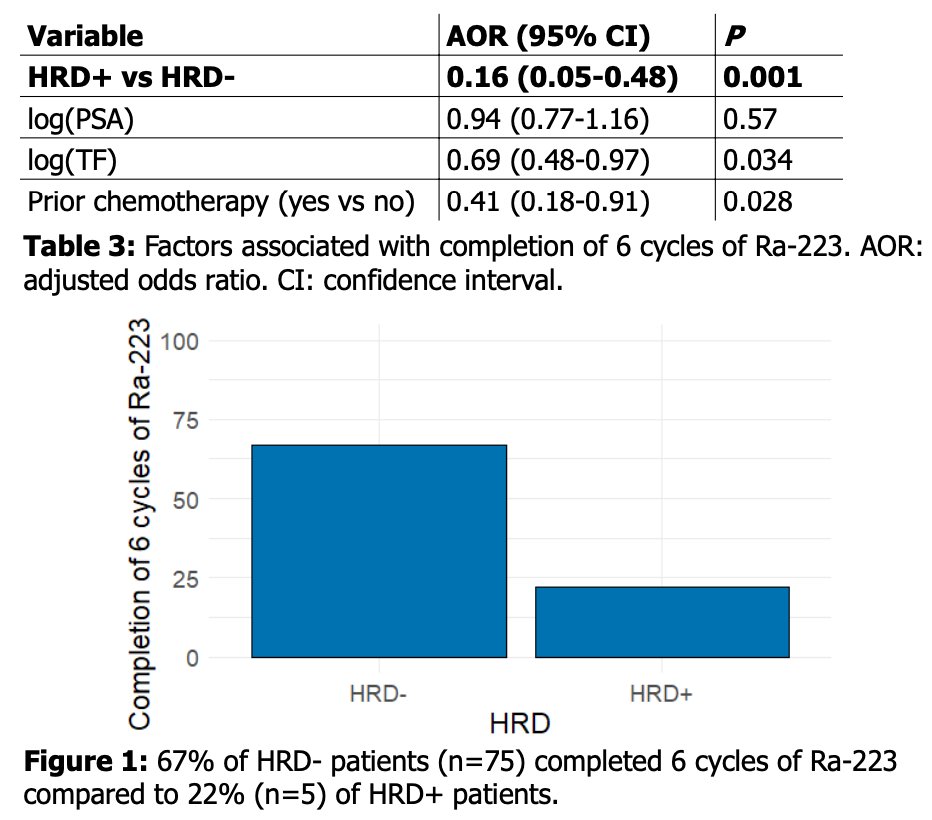
The authors conclude that targeted sequencing on ctDNA for HR deficiency-associated genes detected similar frequency of HRD, as compared to prior tissue-based assays, despite a low median tumor fraction. Presence of putative HRD was associated with receipt of fewer than 6 cycles and the authors report no identified confounders and, significantly, these data suggest that patients harboring deleterious alterations in HR associated genes do not appear to garner greater benefit from the double-stranded breaks conferred by radium-223. Ongoing ctDNA and subsequent validating tissue-based studies are planned. Future integration of mutational signature associated with HRD may refine the phenotypic consequence of the detected mutations.
Presented by: David D. Yang, MD, Department of Radiation Oncology, Brigham and Women’s Hospital/Dana-Farber Cancer Institute, Boston, MA, USA
Written by: Jones Nauseef, MD, PhD, Assistant Professor of Medicine within the Division of Hematology and Medical Oncology, Sandra and Edward Meyer Cancer Center, and Englander Institute for Precision Medicine Weill Cornell Medicine and Assistant Attending physician at NewYork-Presbyterian Hospital. @DrJonesNauseef on Twitter during the 2023 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, Thursday Feb 16 – Saturday Feb 18, 20223


