(UroToday.com) The foundational therapy for metastatic prostate cancer remains inhibition of androgen receptor (AR) signaling, yet nearly all patients will develop resistance to castration, known as castration resistant prostate cancer (CRPC). Latency to CRPC is highly variable and it is those patients with early development of CRPC or transformation into neuroendocrine prostate cancer (NEPC) who have the poorest prognosis. Early detection of the transformation to CRPC or NEPC could identify those patients who may need treatment intensification or alterative therapies. As yet, the ability to do so is limited.
Dr. Amy Taylor and colleagues sought to use their established method for performing RNA sequencing on isolated EpCAM-positive circulating tumor cells (CTCs) to address this unmet need. As previously reported and schematized below, this approach allows non-invasive multiplex serial evaluation of prostate cancer with clinical outcome associations1,2.
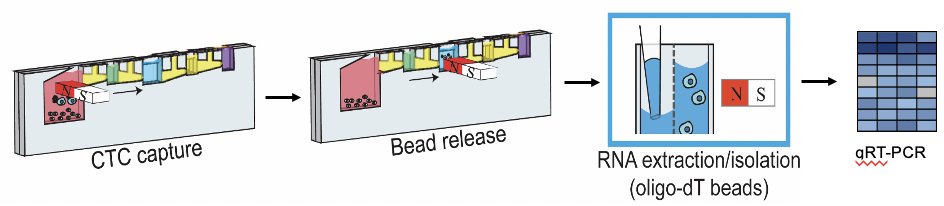

Under IRB approval, CTCs were isolated from 99 patients with castration sensitive (CSPC), castration resistant (CRPC), and neuroendocrine prostate cancers (NEPC). Expression of KLK2, KLK3 (PSA), TMPRSS2, FOLH1 (PSMA), synaptophysin (SYP), and chromogranin (CHGA) was measured. Samples were binned by presence or absence of AR- and NE-associated pathway genes. Samples were considered AR+ if ≥3 AR pathway genes (TMPRSS2, KLK2, KLK3, and FOLH1) were positive and were considered NE+ if either or both SYP or CHGA were positive. Samples could be both AR+ and NE+, consistent with known amphicrine disease.
Patient characteristics (Table) demonstrated a balance of de novo and treatment-emergent metastatic disease, a majority of CRPC cases, and predominance of bones and lymph nodes as sites of disease. Approximately half (48%) of subjects had received an AR pathway inhibitor prior to collection.
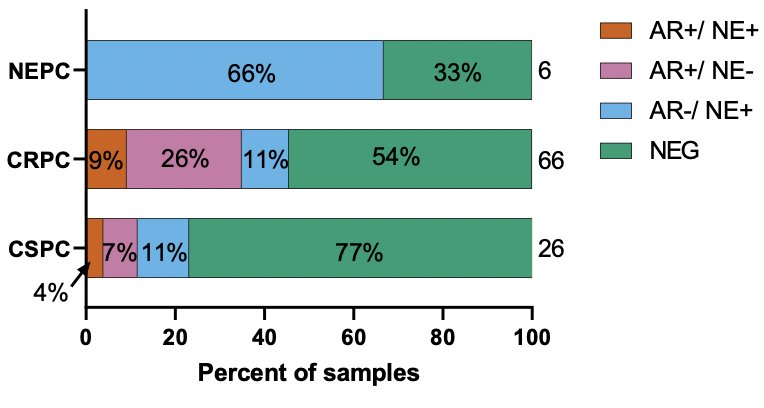
Individual patient level AR/NE-associated gene expression with associated clinical state and overall survival was provided in the presentation. Samples from patients with NEPC were enriched in CTC+ cases, but without any AR+ markers. CSPC, in contrast, was represented by a majority of Negative cases (bar plot, right).

Among all patients, those with either AR+ and/or NE+ CTCs had a significantly shorter median overall survival (OS, 26 vs 10 months, HR 2.29, CI 1.37-3.82, p=0.0002) as depicted in the top Kaplan-Meier curve. In the subset of patients with CRPC, those with AR+ and/or NE+ were found to have a median OS of 6.85 months vs 18.1 months in the AR- and NE- group (HR 2.08, CI 1.20-3.59, p=0.0025). The authors also evaluated for differences in the established non-invasive clinical marker (serum PSA) between disease states and CTC-derived AR/NE status. No difference in median PSA was observed between the positive and negative CTC patients. Most relevantly in the group containing all CRPC and NEPC patients, PSA was significantly lower in the AR-/NE+ group as compared each to the AR+/NE+ and AR+/NE- groups.

The authors summarized the conclusions of their exciting work by stating that expression of AR target genes, as detected in liquid biopsies, is associated with worse OS duration, irrespective of presence of NE features. Expression of NE features with or without AR-positivity, may anticipate the transition to NEPC. Finally, lower PSAs were associated with the development of NE-positivity. As future directions, the authors are pursuing CLIA certification to establish their assay as a clinical grade liquid biopsy assay for AR activity and NE differentiation. Paired sequencing may unearth unknown mechanisms of NEPC development for rationale novel clinical trial opportunities. We eagerly anticipate further investigation in larger cohorts, including in NEPC samples from patients treated in the other clinical trials. Continued successes with this work may allow for early detection of NEPC and, if early enough, possibly intervention to reverse or slow its development.
Presented by: Amy K Taylor, MD, Carbone Cancer Center and Department of Medicine, University of Wisconsin, Madison, WI
Written by: Jones Nauseef, MD, PhD, Assistant Professor of Medicine within the Division of Hematology and Medical Oncology, Sandra and Edward Meyer Cancer Center, and Englander Institute for Precision Medicine Weill Cornell Medicine and Assistant Attending physician at NewYork-Presbyterian Hospital. @DrJonesNauseef on Twitter during the 2023 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, Thursday Feb 16 – Saturday Feb 18, 20223
References:
- Armstrong AJ, et al. Prospective Multicenter Validation of Androgen Receptor Splice Variant 7 and Hormone Therapy Resistance in High-Risk Castration-Resistant Prostate Cancer. J Clin Oncol 37:1120-1129Sperger JM, et al. Prospective Evaluation of Clinical
- Outcomes Using a Multiplex Liquid Biopsy Targeting Diverse Resistance Mechanisms in Metastatic Prostate Cancer. J Clin Oncol. Sep 10 2021;39(26):2926-2937.


