(UroToday.com) The 2023 American Society of Clinical Oncology Genitourinary (ASCO GU) cancers symposium held in San Francisco, CA between February 16th and 18th was host to a Clinical Decision-Making in the Treatment of Localized Prostate Cancer: Controversial Points session. Dr. Joe O’Sullivan presented updated 10-year efficacy and co-morbidity outcomes of CHHiP, a phase III randomized trial of conventional versus hypofractionated high dose intensity modulated radiotherapy for prostate cancer (CRUK/06/016).
Previously published in Lancet Oncology in 2016, the CHHiP trial was a multi-center, randomized, phase III trial, designed as a non-inferiority clinical trial, randomizing men with pT1b-T3aN0M0 prostate cancer and PSA ≤30 ng/ml (risk of SV involvement ≤30% per Rotterdam Formula) 1:1:1 to conventional (74 Gy delivered in 37 fractions over 7.4 weeks) or one of two moderate hypofractionated schedules (60 Gy in 20 fractions over 4 weeks or 57 Gy in 19 fractions over 3.8 weeks) all delivered with intensity-modulated techniques. Randomization was stratified by NCCN risk group, with low-, intermediate-, and high-risk patients included. Most patients (97%) were given radiotherapy with 3-6 months of neoadjuvant and concurrent androgen suppression. The primary endpoint was time to biochemical or clinical failure, and the critical hazard ratio for non-inferiority was 1.208. In this large trial, 3,216 men were enrolled from 71 centers and randomly assigned, as follows: 1,065 patients to the 74 Gy group, 1,074 patients to the 60 Gy group, and 1,077 patients to the 57 Gy group.1
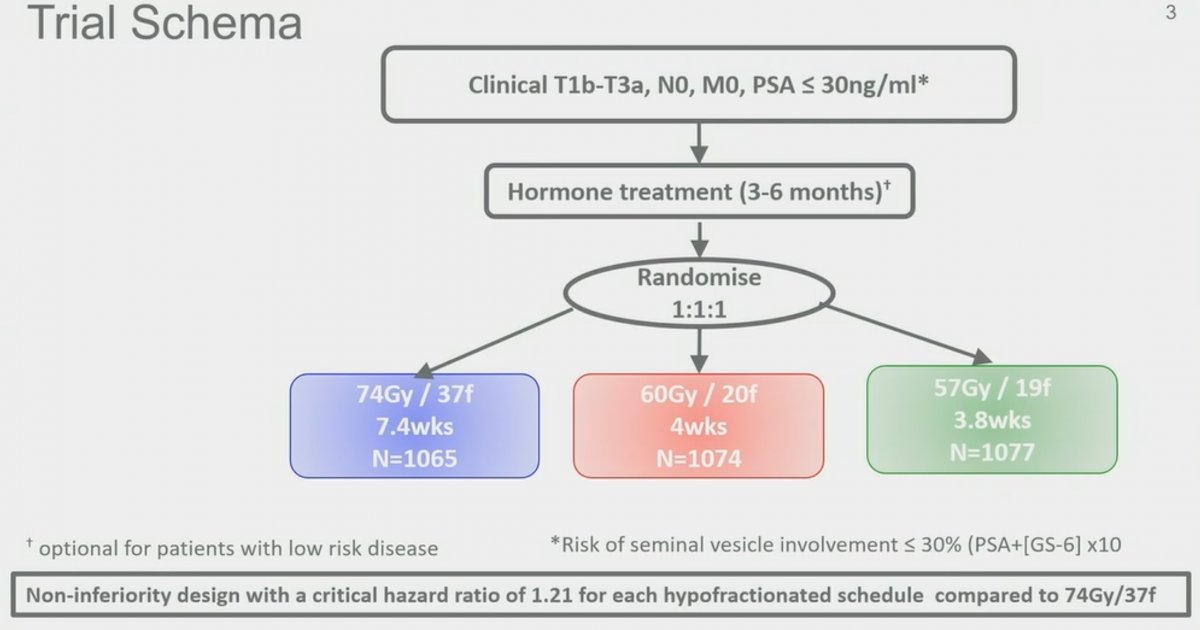
The median age of the study participants was 69 (IQR: 64 – 73), with 73% of patients having NCCN intermediate risk cancer and 43% having Grade Grape 2 disease. The median PSA was 10 ng/ml (IQR: 7 to 14). At the time of publication in 2016, the cohort had a median follow-up of 5.2 years, with 417 primary endpoint events having occurred. Dr. O’Sullivan presented updated results as of January 2023, with now 12.1 years of median follow-up and 772 primary endpoint events having occurred. As demonstrated in the Kaplan Meier curve below, the 10-year event-free rates for each of the three arms were:
- 74 Gy: 76% (95% CI: 73.1 - 78.6%)
- 60 Gy: 79.8% (95% CI: 77.1 – 82.3%)
- 57 Gy: 73.1% (95% CI: 70.2 – 75.9%)
Based on the above outcomes, Dr. O’Sullivan inferred that the 60 Gy hypofractionated schedule remains non-inferior to 74 Gy conventional fractionation. However, it appears that the 57 Gy hypofractionated schedule is inferior, with respect to biochemical failure/PCa recurrence outcomes, compared to 74 Gy conventional fractionation
Given the demonstrated non-inferiority, the investigators proceeded to perform superiority analysis, which failed to demonstrate a superiority for 60 Gy versus 74 Gy regimens (HR: 0.86 favoring hypofractionation, 95% CI: 0.71 – 1.02, p=0.089) or 57 Gy versus 74 Gy (HR: 1.13, 95% CI: 0.96 – 1.34, p=0.144). Notably, the 60 Gy hypofractionated regimen was significantly superior to the 57 Gy regimen (HR: 0.76 in favor of 60 Gy, 95% CI: 0.64 – 0.90, p=0.002). Dr. O’Sullivan highlighted that it appears that the additional 3 Gy dose is critical for maximizing oncologic outcomes in this patient cohort.
Ten-year metastasis-free survival rates were excellent in all three treatment arms, with rates of 93.0% to 94.3%, as demonstrated below.
Similarly, 10-year overall survival rates were non-significantly different between the three treatment arms with rates of 78.4% to 83.0%. The causes of death were:
- Prostate cancer: 15%
- Second cancer: 23%
- Other: 42%
- Unknown: 20%

With regards to urinary bother at five years (previously reported), moderate/big bother at 5 years were present as follows:
- 74 Gy: 6.7%
- 60 Gy: 9.3%
- 57 Gy: 7.9%
Late bladder toxicity (6-10 years after radiotherapy), as measured by need for TURP, urethrotomy, urethral dilation, long-term catheter, or ureteric obstruction were n the range of 1-2% only:

With regards to bowel bother at five years (also previously reported), moderate/big bother at 5 years were present as follows:
- 74 Gy: 5.4%
- 60 Gy: 7.6%
- 57 Gy: 5.3%
Late bowel toxicity (6-10 years after radiotherapy) was similarly low. As demonstrated below, the need for sigmoidoscopy was the greatest “driver” of bowel toxicity, with 10.6% of patients undergoing a sigmoidoscopy. Other GI complications such as bowel strictures, need for steroids, sucralfate, formalin, laser coagulation, and rectal diversion were all <1% each,

One notable strength of this trial is the minimal loss to follow-up, with 2,395 (94%) of the 10-year co-morbidity forms received from the trial participants.
Dr. O’Sullivan concluded as follows:
- Long-term follow-up confirms that hypofractionation with 60Gy/20f is non-inferior to 74Gy/37f
- Updated 10-year results support continued use of 60Gy/20f as standard of care for men with localized prostate cancer
- Late comorbidities were very low across all treatment groups
- Ongoing work to validate late co-morbidity data collection through comparison with routinely collected health records data are ongoing
Presented by: Joe M. O’Sullivan, MD, FRCP, FFRRSCI, FRCR, Clinical Professor, Radiation Oncology, Queen’s University Belfast, Belfast, Ireland
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, San Francisco, CA, February 16th – February 18th, 2023
Reference:

