(UroToday.com) The 2023 American Society of Clinical Oncology Genitourinary (ASCO GU) cancers symposium held in San Francisco, CA between February 16th and 18th was host to a Clinical Decision-Making in the Treatment of Localized Prostate Cancer: Controversial Points session. Dr. Nicholas van As presented the primary endpoint results from PACE-A: an international phase III randomized controlled trial comparing stereotactic body radiotherapy (SBRT) to surgery for localized prostate cancer.
Dr. von As began by acknowledging that men presenting with early stage localized prostate cancer have several treatment options. Furthermore, the great majority of men treated radically for localized prostate cancer will not die of their disease. As such, treatment-related toxicity is therefore important in decision making. To date, PACE-A is the first randomized trial comparing SBRT and radical prostatectomy.
Below is the current schema for the PACE trials:

As highlighted in the figure, patients in PACE-A had low or intermediate risk disease (Gleason Score ≤ 3+4, cT1c-T2c N0-x,M0-x, PSA ≤20 ng/ml), were candidates for surgical therapy, and were subsequently randomized to radical prostatectomy or SBRT (36.25Gy/5F). Toxicity results from PACE-B have previously been published.1
Given the excellent survival outcomes for patients with clinically localized prostate cancer, a major focus of treatment decision-making is treatment-related side effects/complications. As such, PACE-A was designed to evaluate the co-primary endpoints of urinary incontinence (any use of urinary pads) and EPIC bowel bother score at 2 years. Co-primary endpoints were chosen given the expected higher continence in patients undergoing surgery, compared to higher GI symptoms in those undergoing SBRT.
The sample size was driven by the urinary incontinence outcome, with a target sample size of 234 patients at an 80% power and a 2-sided type 1 error rate of 5%. This sample size was calculated using the assumption of 15% incontinence with surgery and 4% with SBRT. This sample size allowed for over 90% power to detect differences in mean EPIC bowel bother subdomain scores of 5 (assuming a standard deviation of 9.4).
This trial enrolled across 10 UK centers, between August 2012 and Feb 2022. As demonstrated below, recruitment stalled at the start of the COVID-19 pandemic (Mar-Apr 2019), and, as such, the independent data monitoring committee (IDMC) recommended trial recruitment cessation, with 123 patients randomized at that time.

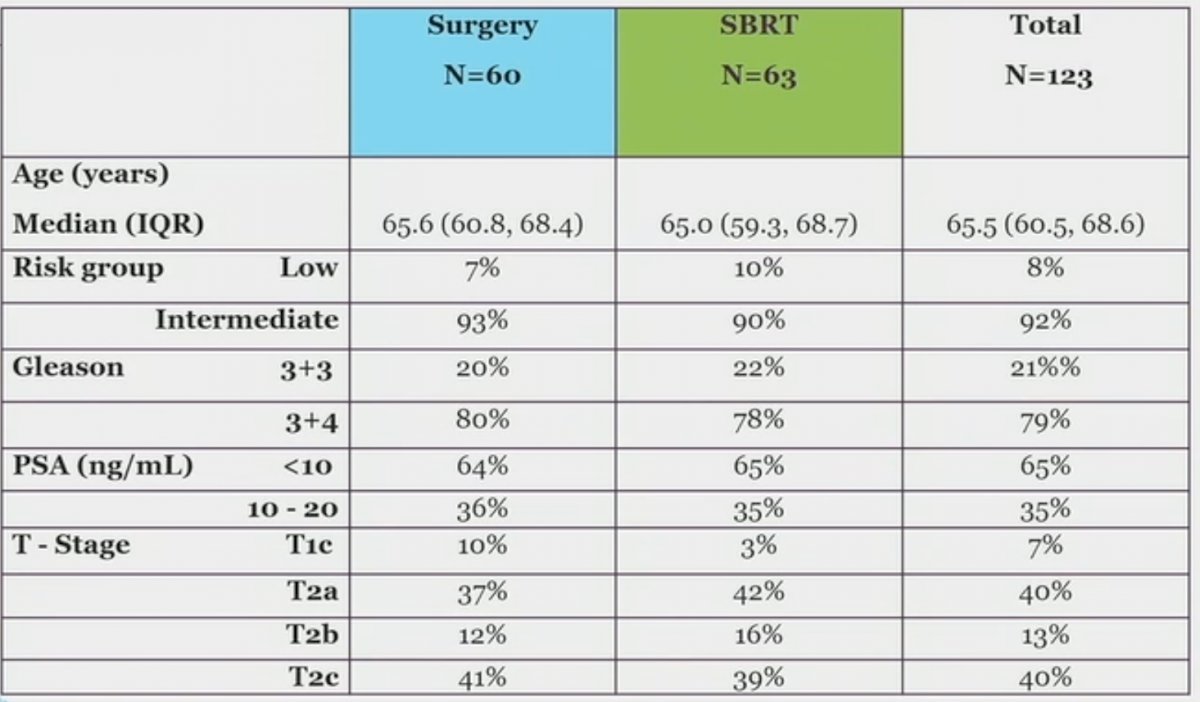
Baseline characteristics are demonstrated below. Of note, 8% of enrolled patients had low-risk disease. 79% had Gleason Score 3+4 disease.
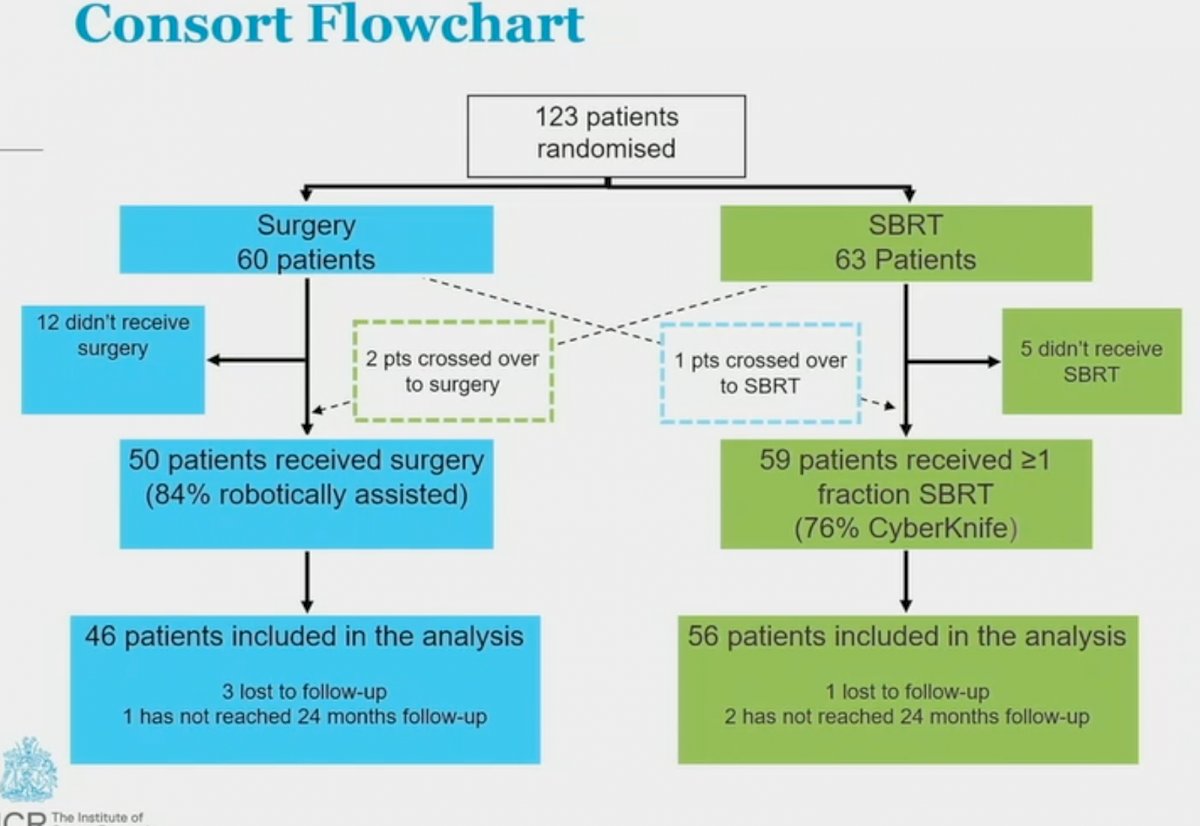
Of the 123 enrolled patients, 60 and 63 were randomized to surgery and SBRT, respectively. Toxicity endpoints were evaluated in patients who actually received the treatment of interest (i.e. per protocol analysis).
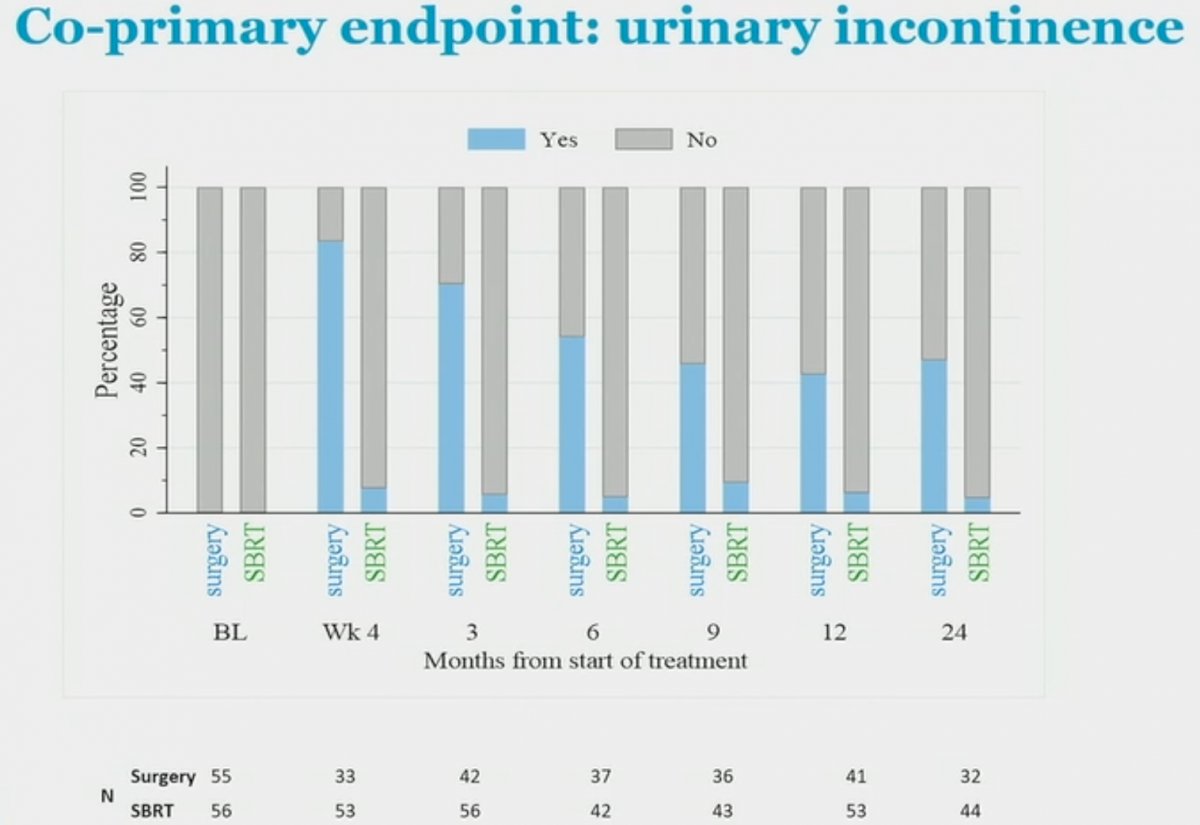
With regards urinary incontinence, the proportion of patients using any urinary pads at 2 years were:
Surgery: 15/32 (47%)
SBRT: 2/44 (4.5%; p for comparison <0.001)
Urinary incontinence in the SBRT group remained relatively stable at the evaluable time points, whereas incontinence in the surgical group gradually improved post-intervention. Of note, across the entire trial cohort, one pad was required in 88% of patients.
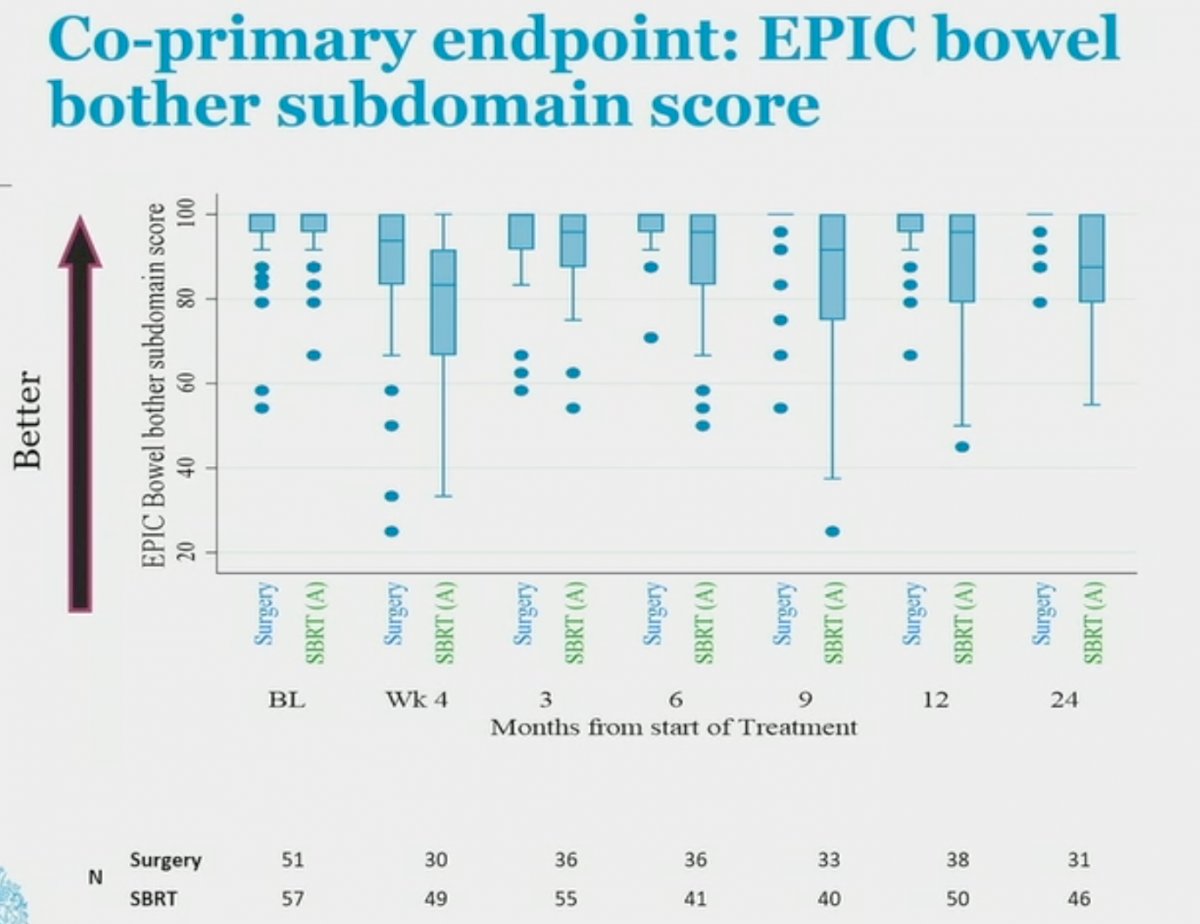
With regards to bowel bother, the mean EPIC bowel bother subdomain score at 2 years was:
- Surgery: 97.3 (SD=5.5)
- SBRT: 88.7 (SD=12.7; p for difference <0.001)
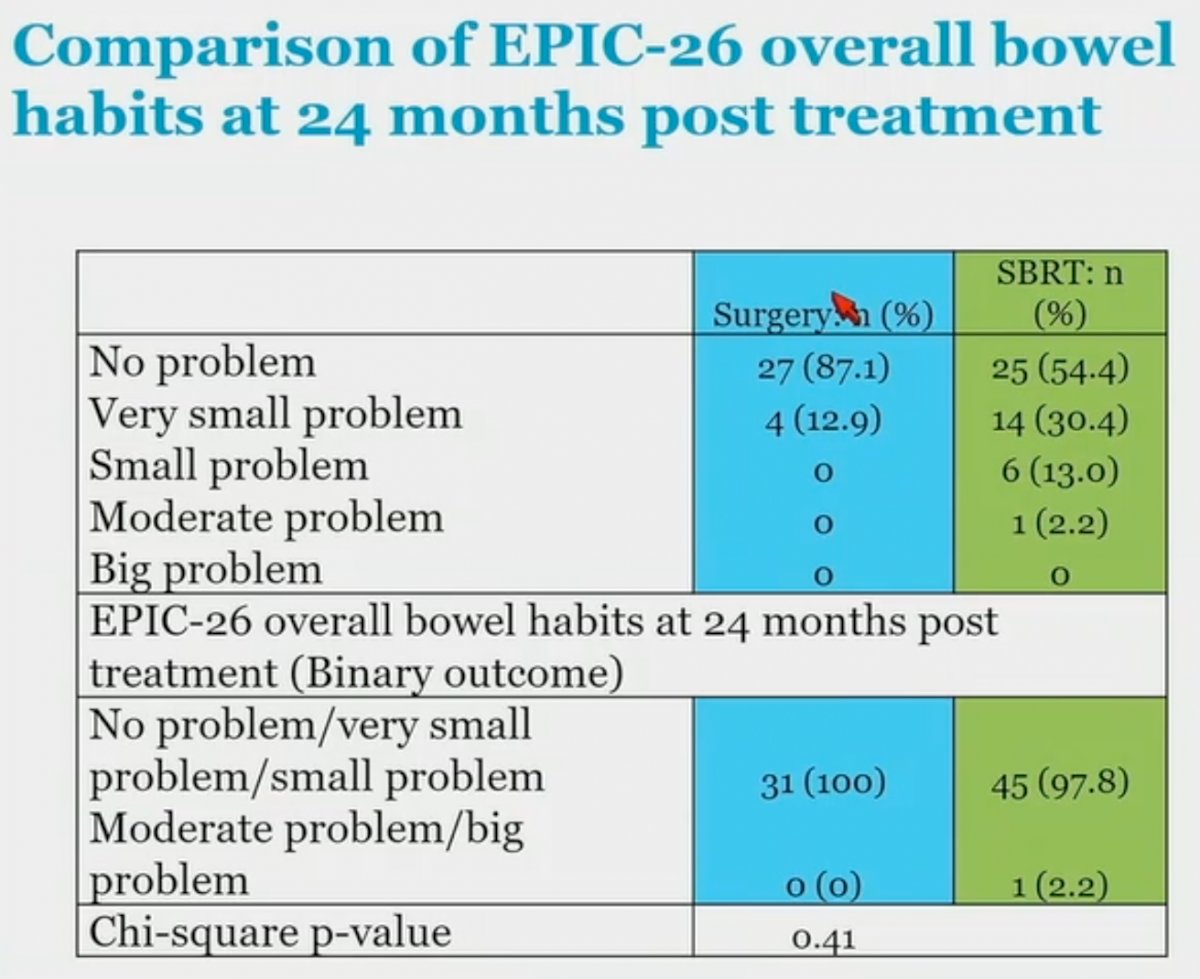
However, when we further analyze the GI symptoms using the EPIC-26 overall bowel habits at two years post-treatment, we note that only 1 patient (2.2%) in the SBRT arm reported having moderate or big problems.
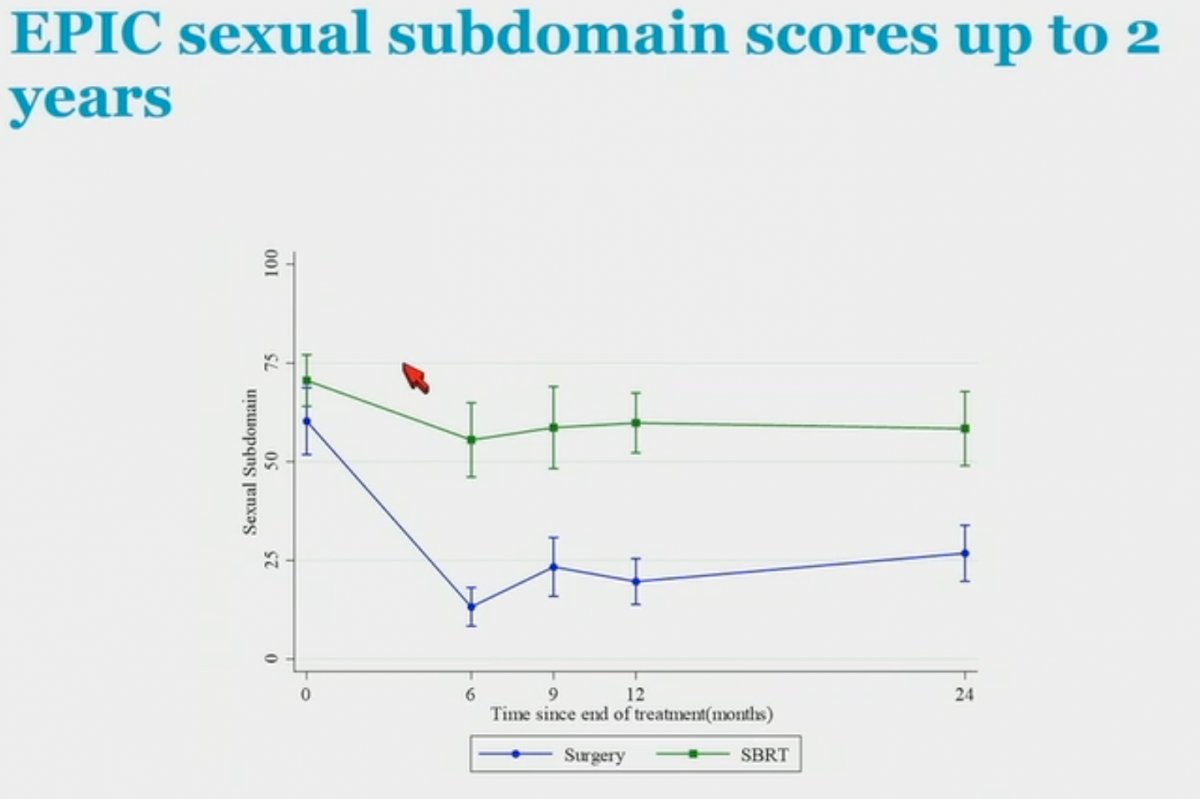
With regards to sexual function, the EPIC sexual bother subdomain score at 2 years was better in the SBRT arm (57.7 versus 29.3 for surgery; p<0.001).
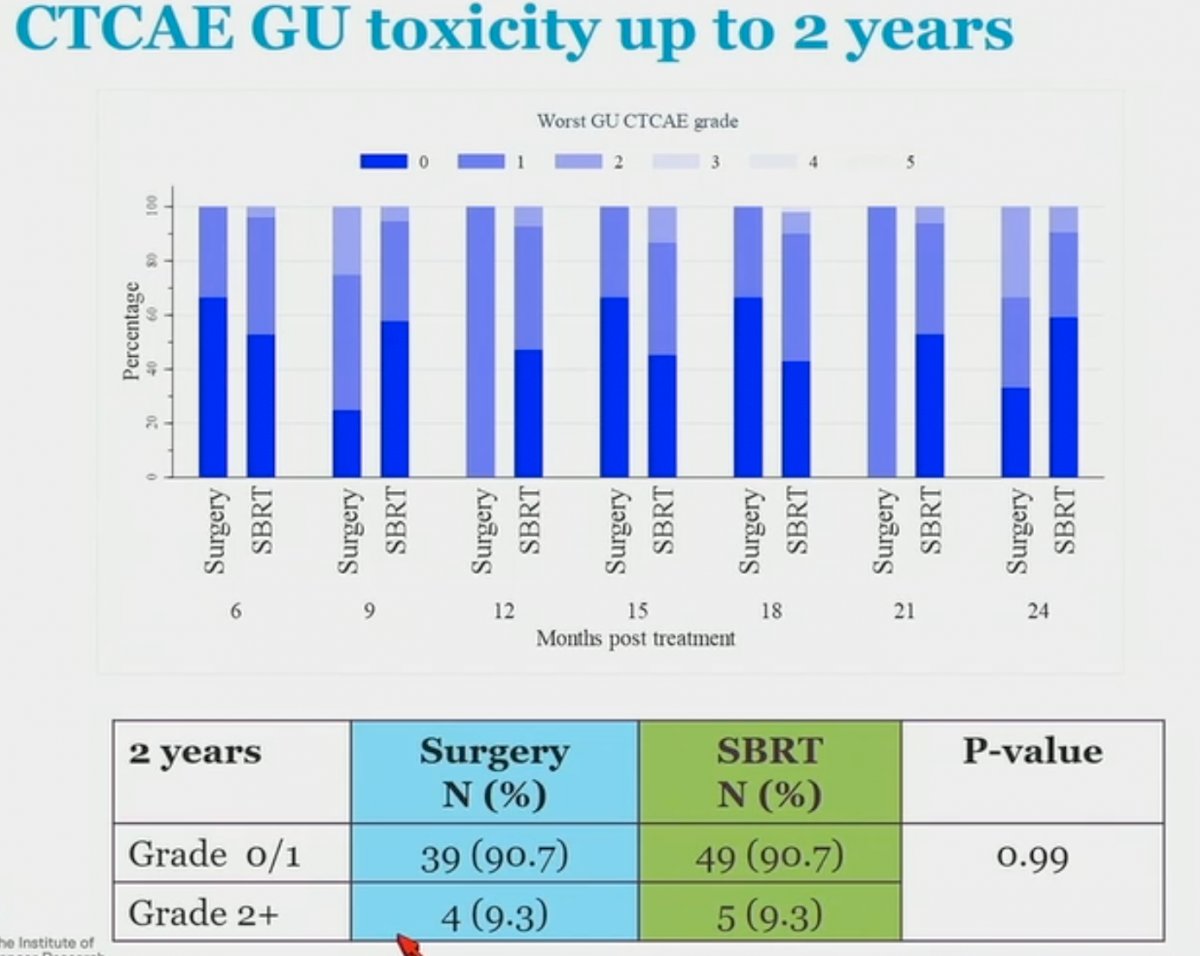
Interestingly, when GI toxicity was assessed by clinicians (CTCAE) at two years, there were no significant differences between the two arms with all patients in both arms having Grade 0 – 1 toxicity only. Similarly, there were no significant differences in clinician-assessed GU toxicity between the two arms at two years. As such, one of the strengths of this trial was the measurement of both patient-reported and physician-reported outcomes and highlighting the measurement bias associated with clinician reported outcomes.

Dr. von As concluded as follows:
- Compared with surgery, patients receiving SBRT reported better urinary continence and less sexual bother at 2 years
- SBRT patients reported bowel bother symptoms at 2 years than surgery patients
- Grade 2 or worse CTCAE (clinician-reported outcomes) were rare in both arms
- Efficacy data will be reported in the Summer of 2023 (5-year follow-up)
Presented by: Nicholas John von As, MBBCh, MRCP, FRCR, Consultant Oncologist, The Royal Marsden NHS Foundation, London, UK
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 Genitourinary (GU) American Society of Clinical Oncology (ASCO) Annual Meeting, San Francisco, Thurs, Feb 16 – Sat, Feb 18, 2023.
References:
- Brand DH, et al. Intensity-modulated fractionated radiotherapy versus stereotactic body radiotherapy for prostate cancer (PACE-B): acute toxicity findings from an international, randomised, open-label, phase 3, non-inferiority trial. Lancet Oncol 2019;20(11):1531-1543.


