(UroToday.com) The 2023 GU ASCO annual meeting included a session on prostate cancer, featuring a presentation by Dr. Loic Mourey discussing results from the PEACE-1 trial, specifically efficacy and safety of abiraterone acetate + prednisone and ADT +/- docetaxel in older patients (≥70 years), with de novo metastatic-castration sensitive prostate cancer (mCSPC), compared to younger patients (<70 years). Previously, the PEACE-1 trial1 showed that adding abiraterone acetate + prednisone to ADT +/- docetaxel in mCSPC improved overall survival and radiographic progression free survival, with a modest increase in toxicity (mostly hypertension). Because mCSPC primarily affects older men, this post-hoc analysis investigated the safety and efficacy of abiraterone acetate + prednisone stratified by age in PEACE-1.
Men with de novo mCSPC were allocated to standard of care versus standard of care + abiraterone acetate + prednisone versus standard of care + radiotherapy versus standard of care + abiraterone acetate + prednisone + radiotherapy in this 2x2 design phase 3 trial. Standard of care was initially ADT alone, then from October 2015 onwards, the use of docetaxel was authorized as part of standard of care (at the investigator’s discretion until 2017, then, following the publication of LATITUDE and STAMPEDE trials, accrual was restricted to men receiving ADT + docetaxel). Efficacy and safety in older men included in PEACE-1 were analyzed with the same methods used in the overall trial.
A total of 741 younger men (63%) and 431 older men (37%) were randomized. Older men presented with more altered performance status (ECOG 1-2) (36% vs 26% p=0.0003) and less frequent use of docetaxel as part of standard of care (66% vs 51% p<0.0001) than younger men. Hypertension (56,5% vs 38,2%, p<0.001) and diabetes mellitus type 2 (15.5% vs 11%, p=0.029) were significantly more frequent in older men. Median time to abiraterone acetate + prednisone discontinuation was shorter (30.0 months [95% CI 22.1, 35.4] vs 41.4 [95% CI 31.5, 54.0] independently of docetaxel use and more frequently due to adverse events or death in the older than younger population. The benefit of abiraterone acetate + prednisone on radiographic progression-free survival (rPFS) tended to decrease with age in the overall population: HR 0.65, 95% CI 0.42-1.01 in older men vs HR 0.49, 95% CI 0.35-0.69 for younger men:
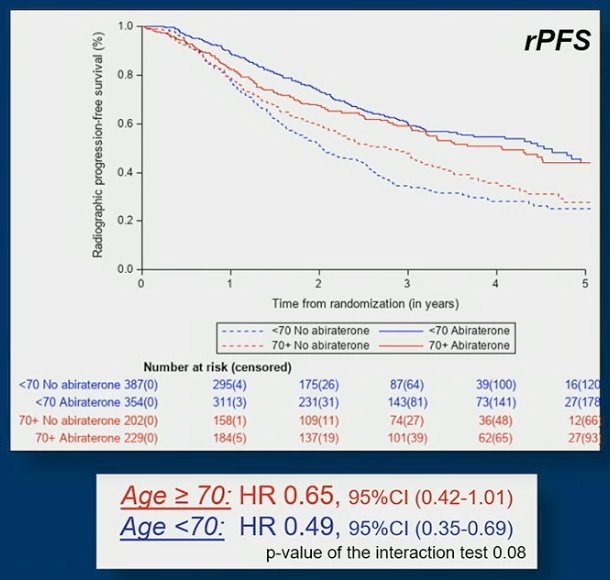
The same trend was observed on overall survival (OS): HR 0.95, 95% CI 0.72-1.25 for older men vs HR: 0.73, 95% CI 0.58-0.92 for younger men:
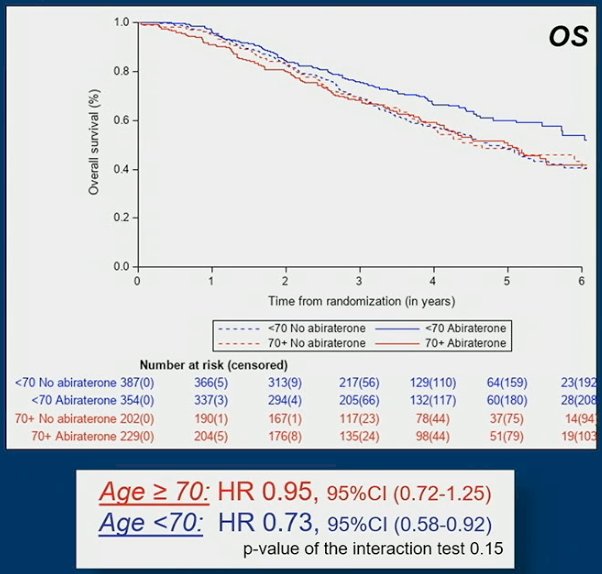
However, in men fit to receive the standard of care composed of ADT + docetaxel, the rPFS benefit of abiraterone acetate + prednisone was comparable in older men (HR 0.55, 95%CI 0.29-1.04) and in younger men (HR 0.50, 95%CI 0.33-0.78):
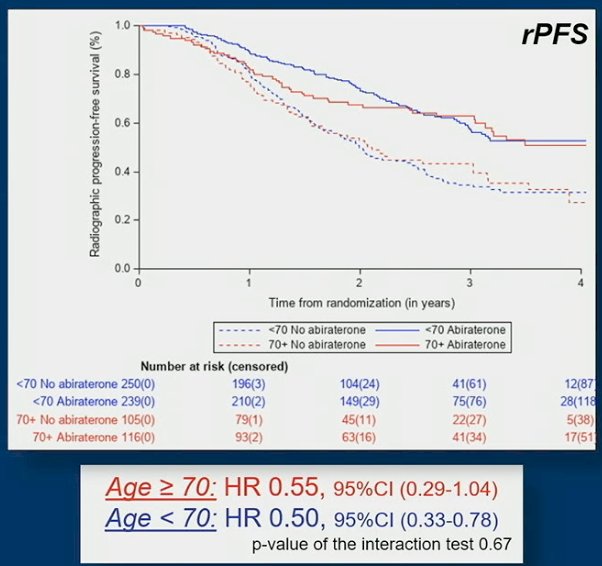
The OS benefit of abiraterone acetate + prednisone was: HR 0.80, 95%CI 0.53-1.20 for older men and HR 0.71, 95%CI 0.52-0.95 for younger men:
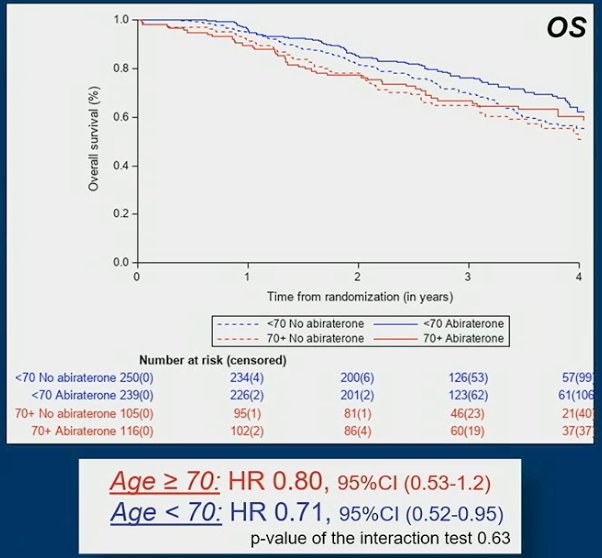
Safety data showed that severe adverse events (grade 3-5) were more frequent in older men receiving abiraterone acetate + prednisone in comparison with younger men (69% vs 61%) while there was no difference between older and younger men not receiving abiraterone acetate + prednisone (48% vs 47%).
Dr. Mourey concluded this presentation discussing results from the PEACE-1 trial, specifically efficacy and safety of abiraterone acetate plus prednisone and ADT +/- docetaxel in older patients (≥70 years), with de novo mCSPC, compared to younger patients (<70 years) with the following concluding messages:
- In the overall population, older men derive a lower benefit, both in terms of rPFS and OS, from adding abiraterone acetate + prednisone to standard of care versus younger men
- This decreased benefit is likely due to more toxicity leading to more frequent and earlier drug discontinuation
- Importantly, in older men fit enough to receive ADT + docetaxel, the benefit of adding abiraterone acetate + prednisone to standard of care was comparable to younger men
- In men older than 70 years of age considered for triplet therapy, the recommendations are for careful evaluation (G8 +/- geriatric assessment, comorbidities, polypharmacy), and close follow-up to maintain benefit and preserve quality of life and autonomy
Presented by: Loic Mourey, MD, Institut Universitaire du Cancer-Oncopole, Toulouse, France
Co-Authors: Helen Jane Boyle, Guilhem Roubaud, Raymond S. McDermott, Stephane Supiot, Bertrand F. Tombal, Aude Flechon, Dominik R. Berthold, Philippe Ronchin, Gabriel Kacso, Jean Francois Berdah, Fabio Calabro, Gwenaelle Gravis, Samuel Palumbo, Thierry Gil, Brigitte Vie, Hélène Ribault, Karim Fizazi, Stéphanie Foulon, Joan Carles
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2023 Genitourinary (GU) American Society of Clinical Oncology (ASCO) Annual Meeting, San Francisco, Thurs, Feb 16 – Sat, Feb 18, 2023.
References:
- Fizazi K, Foulon S, Carles J, Roubaud G, et al. Abiraterone plus prednisone added to androgen deprivation therapy and docetaxel in de novo metastatic castration-sensitive prostate cancer (PEACE-1): A multicentre, open-label, randomized, phase 3 study with a 2 x 2 factorial design. Lancet. 2022 Apr 30;399(10336):1695-1707.


