(UroToday.com) The 2023 GU ASCO annual meeting included a session on prostate cancer, featuring a presentation by Dr. Daniel Petrylak discussing results from KEYNOTE-921, a randomized, double-blind, phase 3 study of pembrolizumab plus docetaxel for patients with metastatic castration-resistant prostate cancer (mCRPC). Docetaxel is a treatment option following disease progression on a next-generation hormonal agent for patients with mCRPC, but there is an urgent need for more efficacious treatments. The randomized, double-blind, phase 3 KEYNOTE-921 study (NCT03834506) evaluated the efficacy and safety of pembrolizumab + docetaxel vs placebo + docetaxel for patients with mCRPC who had received prior next-generation hormonal agent therapy.
Eligible patients were ≥18 years old, had mCRPC that progressed on ADT, had received 1 prior next-generation hormonal agent, and had an ECOG performance status of 0 or 1. Patients were randomized 1:1 to receive 200 mg pembrolizumab Q3W or placebo for ≤35 cycles (~2 years) in combination with 75 mg/m2 docetaxel Q3W for ≤10 cycles and 5 mg prednisone BID. The trial design for KEYNOTE-921 is as follows:

The dual primary endpoints were radiographic progression-free survival (rPFS; tested at first interim analysis) per PCWG-modified RECIST 1.1 by blinded independent central review and overall survival (OS; tested at final analysis). The key secondary endpoint was time to initiation of the first subsequent anticancer therapy (at first interim analysis). Safety was one of the secondary endpoints.
Between May 30, 2019 and June 17, 2021, 1,030 patients were randomized to receive pembrolizumab + docetaxel (n=515) or placebo + docetaxel (n=515). The median time from randomization to data cutoff date of June 20, 2022 at final analysis was 22.7 months (range: 12.1−36.7). Baseline characteristics were generally balanced between arms, with approximately half of patients in each arm had received prior abiraterone:
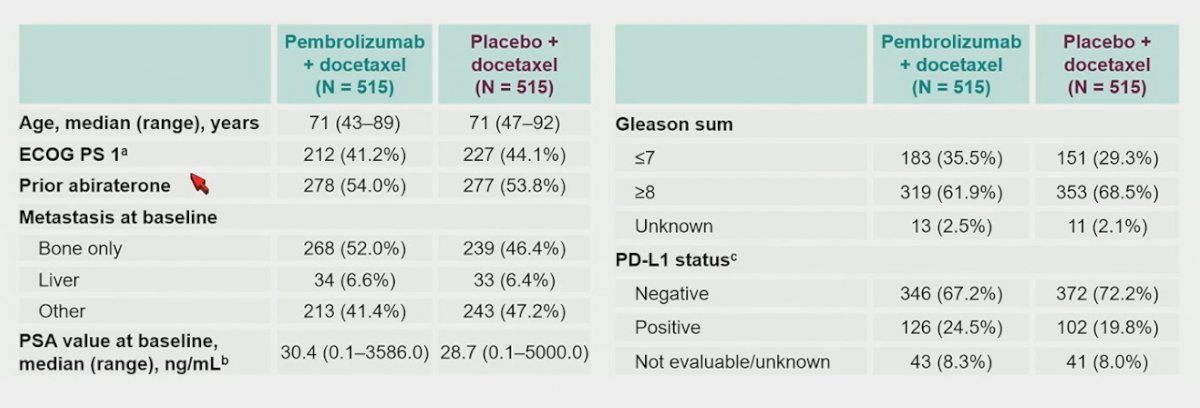
Patients in the pembrolizumab + docetaxel arm received a median of 12 (range: 1–35) cycles of pembrolizumab and 9 (range: 1–12) cycles of docetaxel. Patients in the placebo + docetaxel arm received a median of 12 (range: 1–35) cycles of placebo and 9 (range: 1–10) cycles of docetaxel. The dual primary endpoints of rPFS (median 8.6 months with pembrolizumab + docetaxel vs 8.3 months with placebo + docetaxel; HR 0.85, 95% CI 0.71−1.01):
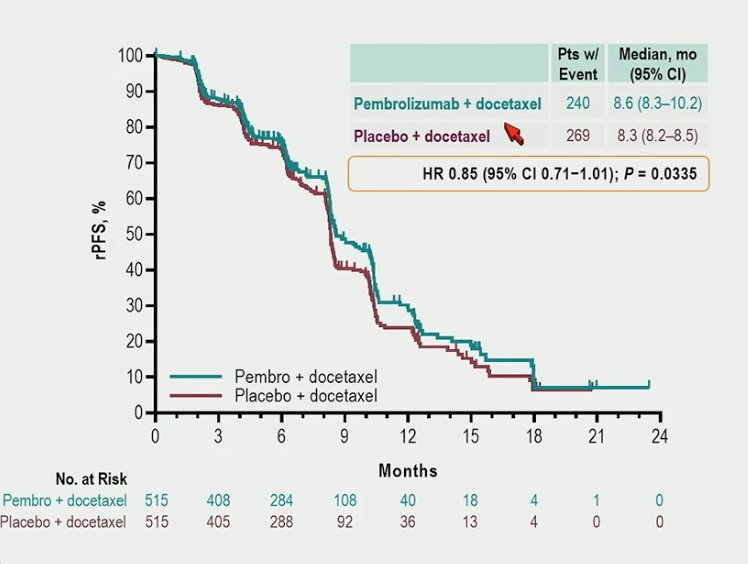
and OS (median 19.6 months vs 19.0 months; HR 0.92, 95% CI 0.78−1.09) were not met:
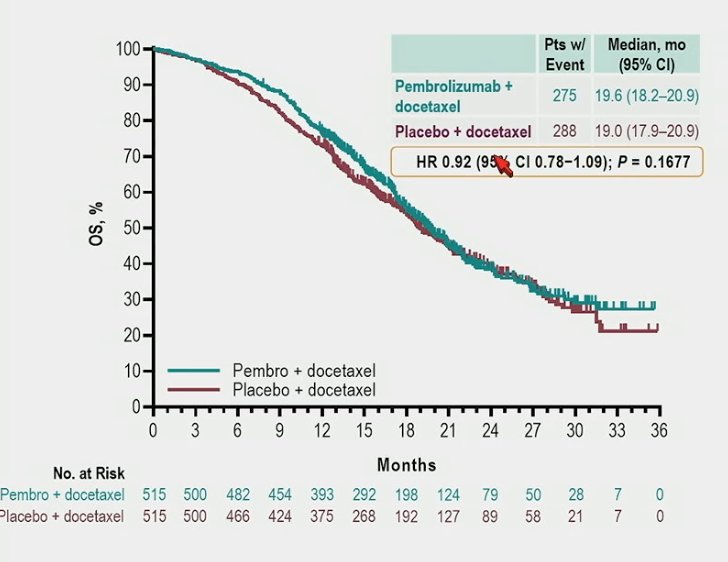
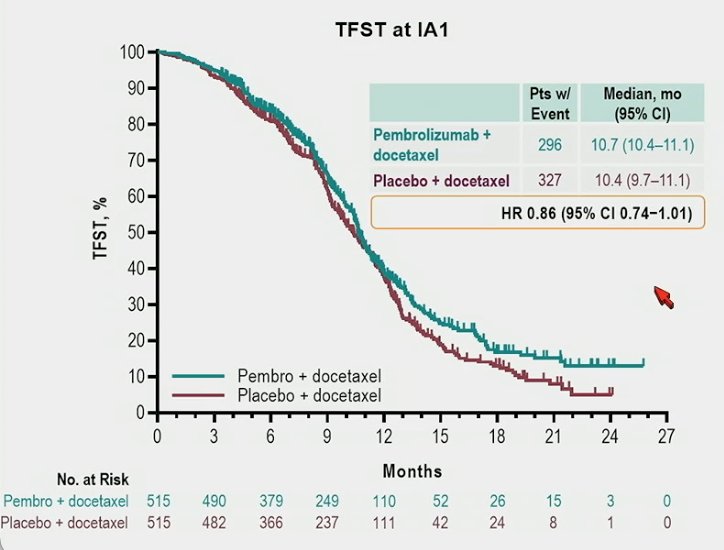
The median time to first subsequent anticancer therapy was 10.7 months vs 10.4 months, respectively (HR 0.86, 95% CI 0.74−1.01):
Treatment-related adverse events occurred in 94.6% (grade ≥3 in 43.2%) and 94.9% (grade ≥3 in 36.6%) of patients with pembrolizumab + docetaxel vs placebo + docetaxel. There were 2 treatment-related deaths with pembrolizumab + docetaxel and 7 with placebo + docetaxel reported. Immune-mediated adverse events and infusion reactions occurred in 23.3% (grade ≥3 in 6.2%) and 12.3% (grade ≥3 in 1.2%) of patients with pembrolizumab + docetaxel vs placebo + docetaxel, most commonly pneumonitis (7.0% vs 3.1%) and hypothyroidism (6.4% vs 3.3%).
Dr. Petrylak concluded his presentation by discussing results from KEYNOTE-921, a randomized, double-blind, phase 3 study of pembrolizumab plus docetaxel for patients with mCRPC with the following concluding messages:
- The addition of pembrolizumab to docetaxel did not significantly improve rPFS or OS for patients with mCRPC
- No notable increases in any-cause or treatment-related adverse events was observed with pembrolizumab + docetaxel vs placebo + docetaxel
- Longer duration of docetaxel treatment was observed in both study arms compared to real-world reports
- The final analysis of KEYNOTE-921 shows the first phase 3 data for a PD-(L)1 inhibitor + docetaxel vs docetaxel for mCRPC. These results do not change the standard of care for patients with mCRPC
- Future studies will be required to determine patient selection and combination strategies to maximize the benefit of anti-PD-1 antibody in prostate cancer
Presented by: Daniel P. Petrylak, MD, Yale Cancer Center, New Haven, CT
Co-Authors: Raffaele Ratta, Nobuaki Matsubara, Ernesto Pablo Korbenfeld, Rustem Gafanov, Loic Mourey, Tilman Todenhöfer, Howard Gurney, Gero Kramer, Andre M. Bergman, Pawel Zalewski, Maria De Santis, Andrew J. Armstrong, Winald R. Gerritsen, Russell Kent Pachynski, Seok-Soo Byun, Xin Tong Li, Charles Schloss, Christian Heinrich Poehlein, Karim Fizazi
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2023 Genitourinary (GU) American Society of Clinical Oncology (ASCO) Annual Meeting, San Francisco, Thurs, Feb 16 – Sat, Feb 18, 2023.


