(UroToday.com) The 2023 GU ASCO annual meeting included a session on prostate cancer, featuring a presentation by Dr. Bridget Koontz discussing results from the phase 3 prospective LIGHTHOUSE study, specifically detection of true positive M1 lesions by 18F-rhPSMA-7.3 PET in newly diagnosed prostate cancer. Radiohybrid (rh) 18F-rhPSMA-7.3 is a novel high affinity PSMA-targeting PET radiopharmaceutical. The LIGHTHOUSE study (NCT04186819) evaluated the diagnostic performance of 18F-rhPSMA-7.3 in newly diagnosed prostate cancer. At the 2023 GU ASCO annual meeting, Dr. Koontz and colleagues reported the 18F-rhPSMA-7.3 verified detection rate, defined as the proportion of patients with M1 lesions identified by blinded image evaluation and subsequently confirmed true positive by biopsy or confirmatory imaging.
Men with treatment-naïve, unfavorable intermediate to very high-risk prostate cancer who were scheduled to undergo radical prostatectomy underwent PET 50-70 min after IV administration of 296 MBq 18F-rhPSMA-7.3. Onsite readers interpreted the images before submission for blinded image evaluation by three central readers. If the onsite read indicated M1 disease, verification (by biopsy, surgery or additional imaging) of PET-positive M1 lesions was attempted prior to treatment. 18F-rhPSMA-7.3 M1 verified detection rate was evaluated among all study patients without major protocol deviations.
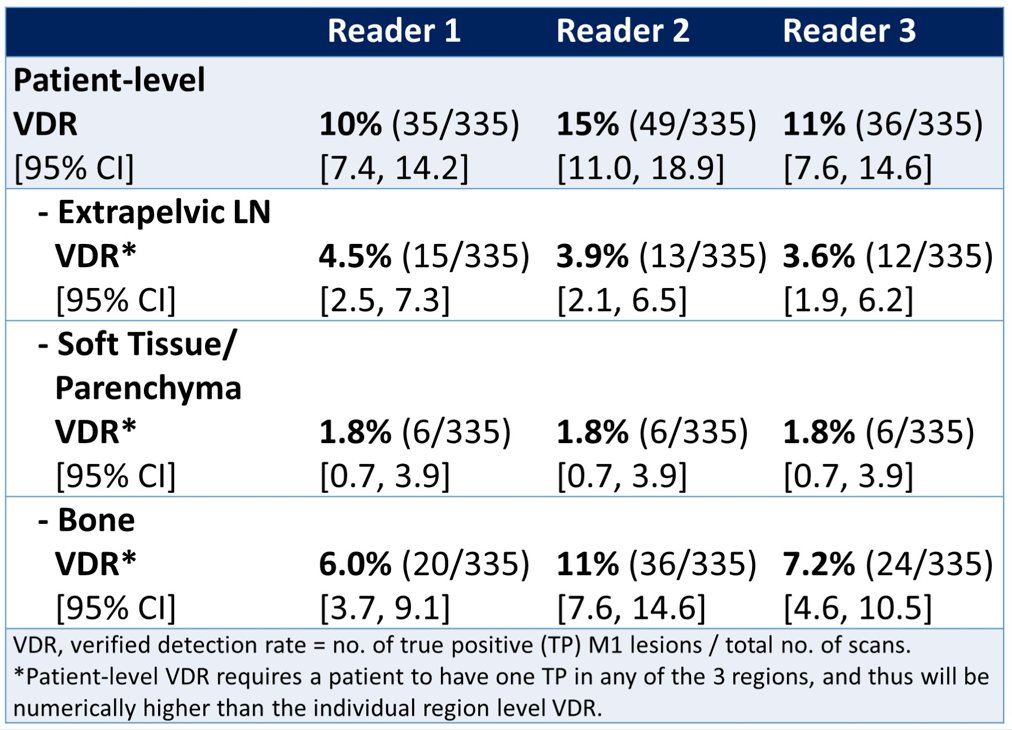
Of the 335 men analyzed (median PSA, 8.89 [range: 1.15-120] ng/mL), 58 (17%) had M1 lesions by majority read. In total, 34 (10%) had verified M1 lesions with individual readers’ M1 verified detection rate ranging from 10-15%:
By region, verified M1 lesions were most common in bone, ranging from 6.0-11% across readers (majority read, 6.6%). Similar data were shown among a subgroup who had negative baseline conventional imaging. In these patients, the verified detection rate ranged from 8.9-13% across readers (majority read, 8.6%). Again, bone showed the highest regional verified detection rate, ranging from 4.8-9.2% (majority read, 5.1%).
Dr. Koontz concluded her presentation discussing results from the phase 3 prospective LIGHTHOUSE study, specifically detection of true positive M1 lesions by 18F-rhPSMA-7.3 PET in newly diagnosed prostate cancer with the following concluding messages:
- Further to its clinically meaningful sensitivity and specificity for pelvic lymph node metastases (reported separately), this study shows 18F-rhPSMA-7.3 PET to be a useful staging tool, as distant metastatic lesions were verified in 10-15% of newly diagnosed patients with unfavorable intermediate to very high-risk prostate cancer
- Determining the presence of M1 disease prior to surgery may help guide treatment planning by identifying patients for whom surgery is the optimal approach, or those for whom alternatives such as radiation therapy and/or androgen deprivation may be more suitable
Presented by: Bridget F. Koontz, MD, Duke University Medical Center, Durham, NC
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2023 Genitourinary (GU) American Society of Clinical Oncology (ASCO) Annual Meeting, San Francisco, Thurs, Feb 16 – Sat, Feb 18, 2023.