(UroToday.com) The 2023 GU ASCO annual meeting included a session on trials in progress for prostate cancer, featuring a presentation by Dr. Jones Nauseef discussing the trial design of a phase I/II dose-escalation study of fractionated 225Ac-J591 for progressive mCRPC in patients with prior treatment with 177Lu-PSMA. As PSMA targeted radiotherapy is now an active standard-of-care treatment in mCRPC, ongoing studies with alternative approaches to targeting PSMA will increasingly need to consider the consequences of sequential PSMA-targeted radiotherapy exposure.
Past and ongoing investigations into antibody-based targeting (e.g., J591) and potent alpha emitting payloads (ie, 225Ac) impact drug kinetics, biodistribution, and resultant clinical toxicities. In a first-in-human phase I dose-escalation study of 225Ac-J591, patients with mCRPC were treated with a single dose of 225Ac-J591 on seven dose levels, up to 93.3 KBq/kg without achievement of maximal tolerated dose. One patient treated at 80 KBq/kg developed dose-limiting toxicity of grade 4 anemia and thrombocytopenia, but 0 of 6 at 93.3 KBq/Kg had grade > 3 hematological toxicity or grade > 2 non-hematological toxicity. Although not intentionally preselected for prior exposure, 55% (12/22) of patients had 177Lu-PSMA previously. With approval of 177Lu vipivotide tetraxetan, Dr. Nauseef and colleagues amended an ongoing phase I dose-escalation study to include a post-177-Lu-PSMA cohort. The hypothesis is that a single fractionated dose-dense cycle of 225Ac-J591 will be safe when given to patients with prior 177Lu-PSMA radioligand therapy.
Entry criteria include progressive mCRPC by PCWG3 criteria, ECOG performance status 0-2, intact organ function, and prior receipt of androgen receptor pathway inhibitor and chemotherapy (or refused/ineligible). There is no limit to prior lines of therapy except alpha-emitting therapies (i.e., PSMA-targeted radiotherapy, radium-223) and in this amended dose-escalation cohort, all patients must have had prior treatment with 177Lu-PSMA. Treatment will be given in a single fractionated cycle of 225Ac-J591 administered on D1 and D15. The phase I component is a 3+3 dose-escalation study design with up to 18 patients, with the goal of identifying maximal tolerated dose. The phase II component will include up to 16-19 patients in a Simon 2-stage design with 90% power to exclude the null hypothesis (35% or fewer patients with PSA50):
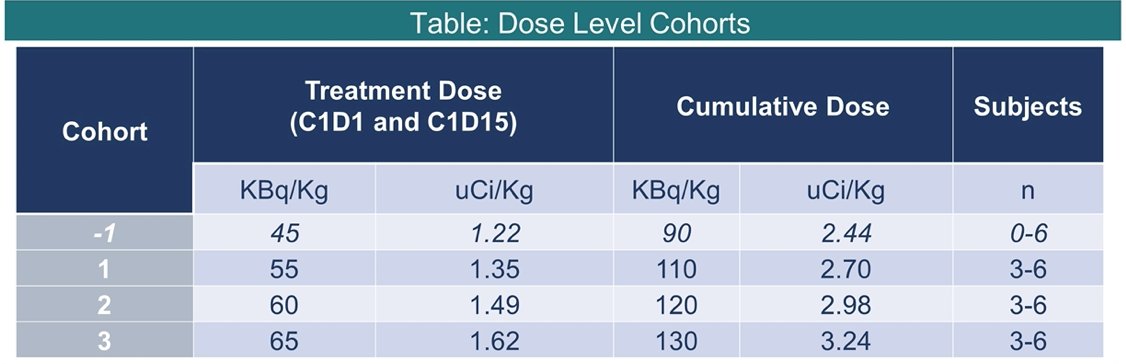
The schema of the trial is as follows:
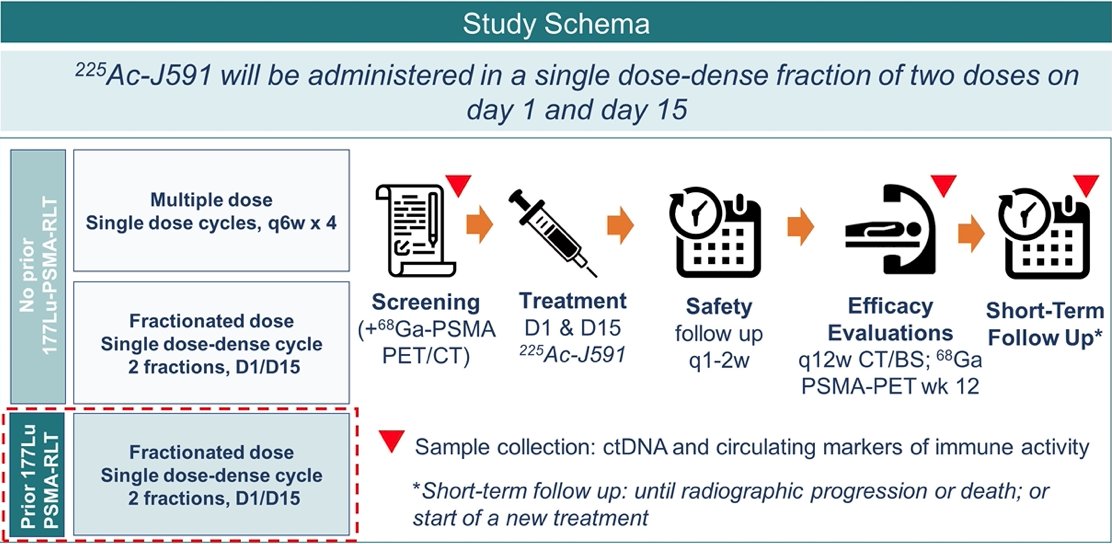
Eligible men with negative PSMA PET scans will be offered treatment with informed consent in an exploratory subgroup but will not be counted towards phase II efficacy. Secondary outcomes include:
- Radiographic response by PCWG3-modified RECIST 1.1 criteria and PSMA PET
- Biochemical and radiographic progression-free survival
- Circulating tumor cell counts
- Overall survival
Patient reported outcomes, genomic, and immune analyses are exploratory. Dr. Nauseef concluded by noting that enrollment to the post-177Lu-PSMA cohort began in August 2022.
Clinical Trial Information: NCT04506567
Presented by: Jones T. Nauseef, Weill Cornell Medicine, Division of Hematology & Medical Oncology; Sandra and Edward Meyer Cancer Center, New York, NY, USA
Co-Authors: Michael Philip Sun, Charlene Thomas, Mahelia Bissassar, Amie Patel, Angela Tan, Escarleth Fernandez, Zachary Davidson, Tessa Chamberlain, Kara Earle, Rebecca Wunder, Sandra Huicochea Castellanos, Peter Gregos, Joseph Osborne, Karla V. Ballman, Ana M. Molina, Cora N. Sternberg, David M. Nanus, Neil Harrison Bander, Scott T. Tagawa
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2023 Genitourinary (GU) American Society of Clinical Oncology (ASCO) Annual Meeting, San Francisco, Thurs, Feb 16 – Sat, Feb 18, 2023.
Related Content:
Fractionated 225Ac-J591 for Progressive mCRPC in Patients with Prior Treatment with 177Lu-PSMA - Jones Nauseef


