(UroToday.com) The 2023 GU ASCO annual meeting included a session on trials in progress for prostate cancer, featuring a presentation by Dr. Arun Azad discussing the trial design of AlphaBet, a phase I/II trial evaluating the combination of radium-223 and [177Lu]Lu-PSMA-I&T (LuPSMA) in patients with metastatic castration-resistant prostate cancer (mCRPC).
LuPSMA is a small molecule radioligand that delivers radiation via beta-particulate emission to cells expressing PSMA. Despite a survival benefit in mCRPC, responses for many are not durable, with the majority of patients developing progressive osseous disease. Due to the lack of crossfire radiation in micrometastatic disease, small cancer cell clusters in the bone marrow may not receive an adequate dose of radiation from 177Lu to induce cytotoxic double-stranded DNA breaks. Alpha-emitters have a shorter path length (≤100 µm) and higher linear energy transfer, making them better suited for treating micrometastatic disease. As a calcium-mimetic, radium-223 is a bone-specific alpha-emitter which targets osteoblastic bone metastases. Dr. Azad and colleagues hypothesize that combining a bone specific alpha emitter with LuPSMA will improve eradication of micrometastatic osseous disease, and thereby lead to higher and longer responses, while being safe and tolerable.
This phase I/II, single-arm, single-centre study will enroll 36 patients with mCRPC who have progressed on a prior second-generation antiandrogen. Up to 6 cycles of LuPSMA (7.4 GBq) and radium-223 (28 kBq/kg - 55 kBq/kg) will be given intravenously every 6 weeks, along with background androgen deprivation and bone protective therapy. Key eligibility criteria include a diagnosis of mCRPC with at least two untreated bone metastases visible on bone scintigraphy and PSMA-positive disease on PSMA PET/CT (SUVmax ≥20). The trial design for this study is as follows:
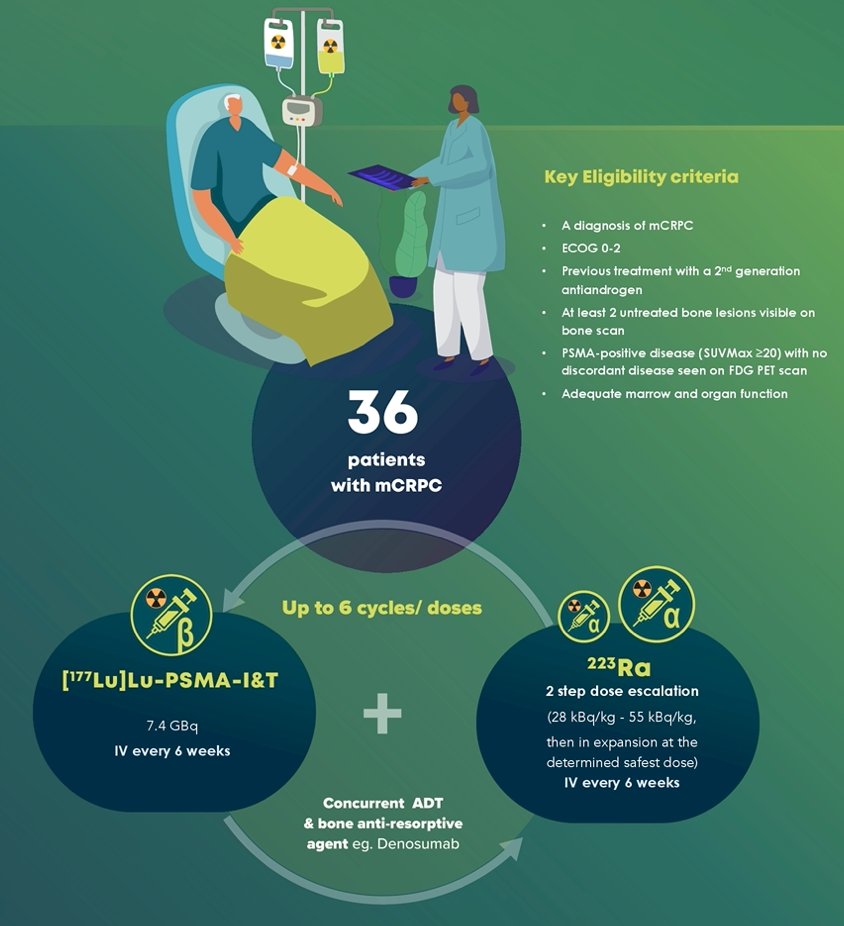
Sites of FDG-positive disease must be either PSMA-positive or have increased uptake on bone scan. Other eligibility criteria include an ECOG status of 0-2, and adequate bone marrow and organ function. Patients treated with more than one line of chemotherapy for metastatic prostate cancer are not eligible. Blood samples and biopsies will be taken at baseline, on treatment, and at progression to develop tumor- and immune-based biomarkers that predict treatment response and associated survival benefit. A traditional 3+3 dose-escalation model will be utilized initially, escalating the dose of radium-223, whereas the dose of LuPSMA will remain fixed. The co-primary objectives of this study are to determine the maximum tolerated dose of radium-223 when combined with LuPSMA, and the 50% PSA response rate. Secondary objectives include:
- Measuring the frequency and severity of adverse events
- Assessment of efficacy through overall, radiographic and PSA progression-free survival
- Overall survival
- Objective tumor response rate
Evaluation of pain and health-related quality of life
Dr. Azad concluded his presentation by highlighting that enrolment commenced in November 2022 and will continue for 24 months.
Clinical Trial Information: NCT05383079
Presented by: Arun Azad, PhD, MBBS, FRACP, Peter MacCallum Cancer Centre and Sir Peter MacCallum Department of Oncology, University of Melbourne, Melbourne, Australia
Co-Authors: Louise Kathleen Kostos, James Patrick Buteau, Theresa Yeung, Sophia Xie, Juliana Di Iulio, Anthony Cardin, Katie Owen, Heidi Fettke, Kwang Y Chin, Brittany Emmerson, Mohammad B Haskali, Belinda Parker, Luc Furic, Michael S Hofman
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2023 Genitourinary (GU) American Society of Clinical Oncology (ASCO) Annual Meeting, San Francisco, Thurs, Feb 16 – Sat, Feb 18, 2023.


