(UroToday.com) The 2023 GU ASCO annual meeting included a session on prostate cancer, featuring a presentation by Dr. Praful Ravi discussing the initial experience with clinical implementation of 177Lu-PSMA-617 at a major academic center. LuPSMA received FDA approval in March 2022 for patients with PSMA-positive metastatic castrate-resistant prostate cancer (mCRPC).
Clinical implementation of this treatment requires multidisciplinary team involvement and has been beset by challenges in drug supply. Dr. Ravi and colleagues established a joint DFCI GU/Nuclear Medicine Tumor Board to review patients for therapy, and at the GU ASCO 2023 annual meeting reported their initial experience.
All patients with mCRPC who had received at least one prior chemotherapy and a novel hormonal agent were considered eligible and referred to tumor board through an online referral system after undergoing PSMA-PET/CT. Case details, including prior treatment history, performance status and organ function, and PET/CT imaging were reviewed at tumor board, with patients either being approved, deferred, or declined for LuPSMA therapy. The LuPSMA referral and tumor board workflow is as follows:
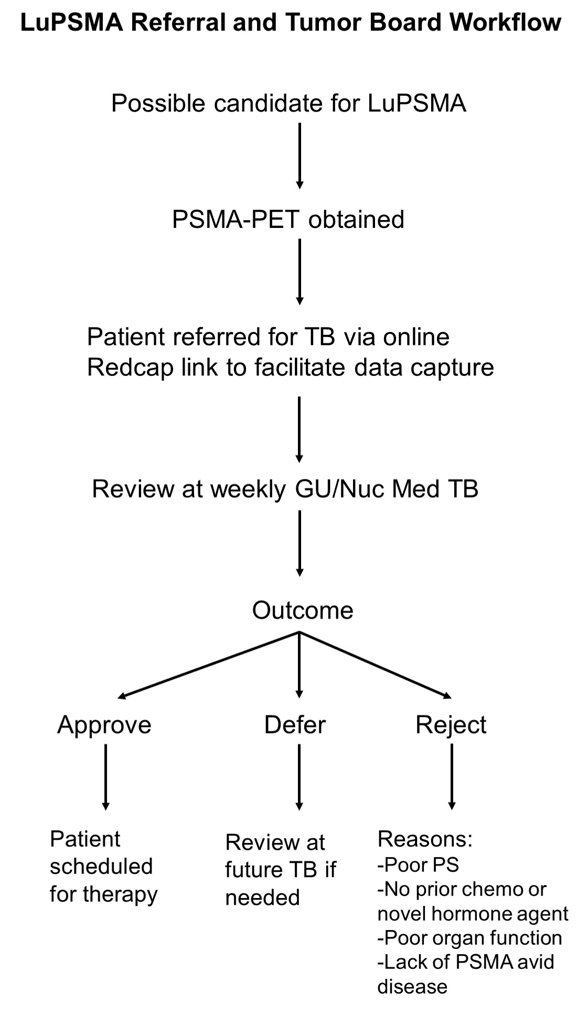
Patients were scheduled for therapy on a first-come first-served basis. Treatment was delivered per standard-of-care at 180-200 millicurie doses every 6 weeks. A questionnaire was sent to 25 referring physicians 2 months after implementation of the tumor board to evaluate the referral process.
Between May 2022 and September 2022, a total of 108 patients were referred for LuPSMA therapy. Median age at time of referral was 73 (range 52-93), and 89% of patients were Caucasian. Median duration between PSMA-PET/CT and tumor board review was 10 days (IQR 6-17). The referred patient demographics are as follows:
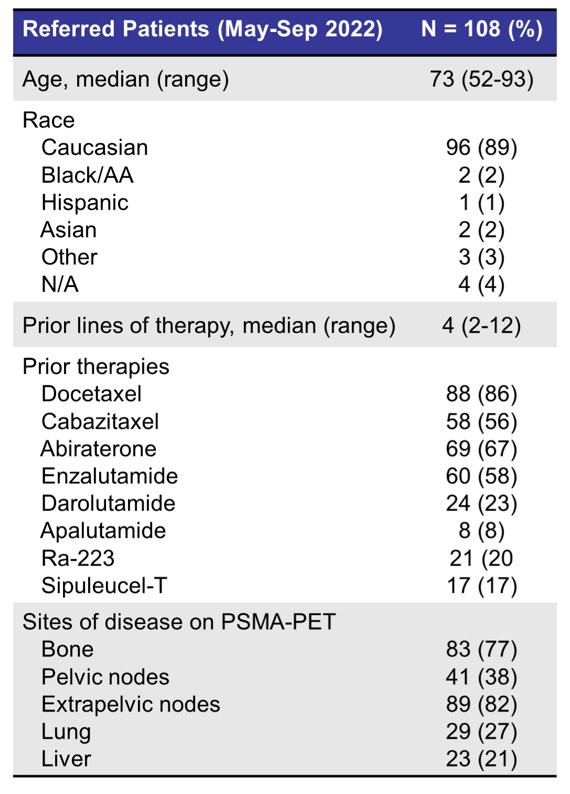
Overall, 84 patients (78%) were approved for therapy, 16 (15%) were deferred and 7 (6%) were declined (reasons including absence of prior chemotherapy, high risk for toxicities, poor performance status); 1 patient died before tumor board review. Prior therapies included docetaxel (86%), cabazitaxel (56%), abiraterone (67%), enzalutamide (58%), darolutamide (23%), radium-223 (20%) and apalutamide (8%). Median number of prior treatments was 4 (range 2-12). Sites of disease on PSMA-PET/CT included bone (77%), pelvic lymph nodes (38%), extrapelvic lymph nodes (82%), lung (27%) and liver (21%). As of September 2022, a total of 40 patients (48%) have received at least 1 cycle of therapy, 17 (20%) have received 2 cycles, and 6 patients (7%) approved for therapy died before receiving 177Lu-PSMA-617. Of the patients that have received 1 cycle of therapy, median duration between tumor board acceptance and C1 was 52 days (range 32-114). Out of 13 survey respondents, all 13 (100%) reported that their overall experience of the referral process was positive or very positive, and 12 (92%) noted that the tumor board had provided additional clinical insights on occasion or frequently.
Dr. Ravi concluded his presentation discussing the initial experience with clinical implementation of 177Lu-PSMA-617 at a major academic center with the following concluding messages:
- Due to drug supply shortages, <50% of patients approved for LuPSMA therapy have started treatment to date
- Median time between tumor board approval and start of therapy was 7-8 weeks, with 7% of patients dying before receiving therapy
- Establishment of a GU/Nuclear Medicine Tumor Board to review cases and facilitate treatment was viewed favorably by treating physicians
Presented by: Praful Ravi, MB, BChir, MRCP, Dana-Farber Cancer Institute, Boston, MA
Co-Authors: Emma Kelly, Bridget Whelpley, Atish Dipankar Choudhury, Rajitha Sunkara, Mark Pomerantz, Mary-Ellen Taplin, Kerry Kilbridge, Xiao X. Wei, Alicia K. Morgans, Renata Rocha de Almeida Bizzo, Andrew Wolanski, Heather Jacene
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2023 Genitourinary (GU) American Society of Clinical Oncology (ASCO) Annual Meeting, San Francisco, Thurs, Feb 16 – Sat, Feb 18, 2023.


