(UroToday.com) The 2024 GU ASCO annual meeting featured a urothelial carcinoma rapid oral abstract session, including a presentation by Dr. Amanda Nizam discussing outcomes in patients with advanced urothelial carcinoma treated with enfortumab vedotin after switch maintenance avelumab in the UNITE study.
Enfortumab vedotin, an anti-Nectin-4 antibody drug conjugate, is approved alone and in combination with pembrolizumab in patients with advanced urothelial carcinoma.1 Maintenance avelumab is approved in patients with advanced urothelial carcinoma without progression on first line platinum-based therapy.2 As patients in the pivotal enfortumab vedotin trials had not received maintenance avelumab after platinum-based therapy, data on outcomes with enfortumab vedotin post-maintenance avelumab are limited. As such, Dr. Nizam and colleagues examined outcomes with enfortumab vedotin post-maintenance avelumab in the multicenter retrospective UNITE study. The hypothesis was that outcomes would be similar to published enfortumab vedotin data.
UNITE is a multicenter, retrospective cohort study of 633 patients with advanced urothelial carcinoma across 16 US sites. Data on enfortumab vedotin outcomes in patient subsets of interest were published in 2021.3 Patients who received sequential platinum-based therapy, maintenance avelumab, then enfortumab vedotin monotherapy were included. Investigator-assessed observed response rate was assessed for evaluable patients with scans after ≥ 1 cycle enfortumab vedotin using χ2 test and logistic regression. Progression-free survival and overall survival were measured from enfortumab vedotin start, and assessed using Kaplan Meier method and Cox proportional hazards model.
Among 633 patients, 49 received platinum-based therapy and maintenance avelumab then enfortumab vedotin. The median age of these patients was 72 years, 63% were men, 96% were Caucasian, 82% were ECOG performance status 0 or 1, 71% had a lower tract tumor, 65% had pure urothelial histology, 71% had visceral or bone metastases, and 33% had a Bellmunt score of 2-3: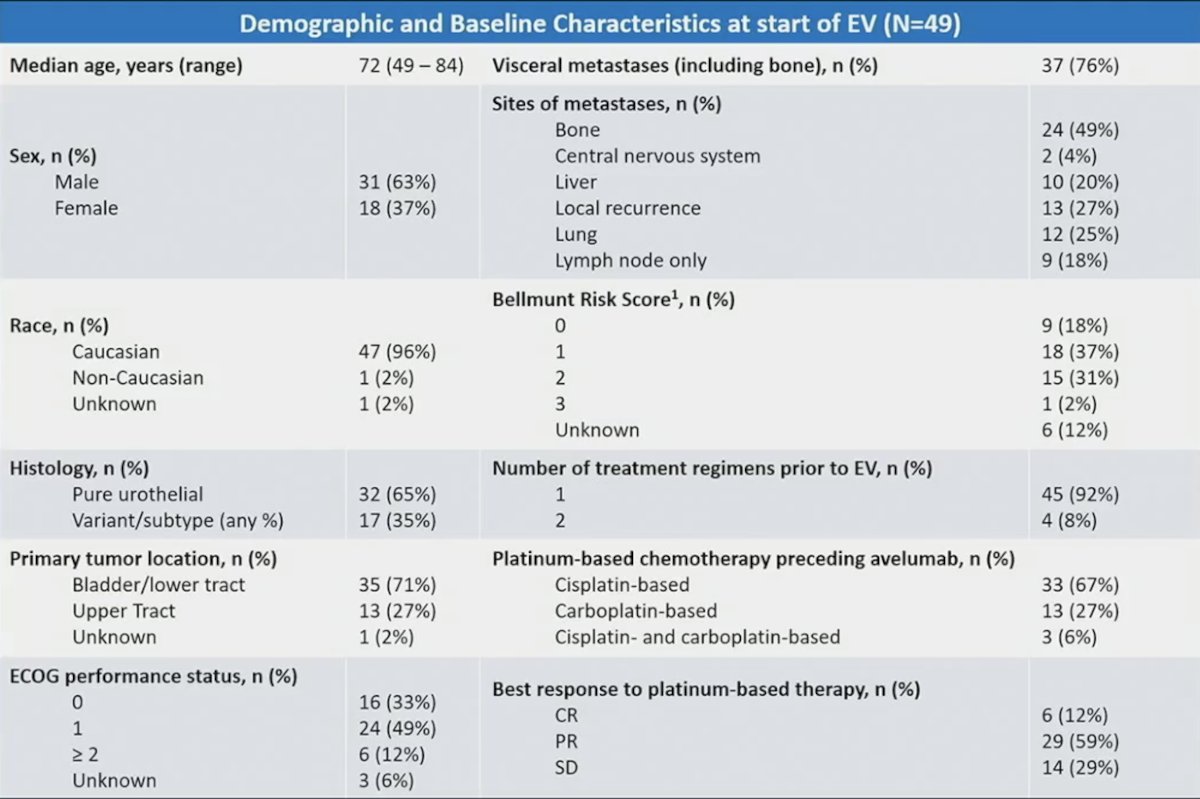
In terms of platinum-based therapy, 67% had cisplatin-based, 26% carboplatin-based, and 6% both cisplatin- and carboplatin-based therapy. Best response to platinum-based therapy was 12% complete response, 59% partial response, and 29% stable disease. The median time from platinum-based therapy start to enfortumab vedotin start was 8.5 months (95% CI 3.9-21.2), and the median follow up from enfortumab vedotin start was 8.5 months (95% CI 6.7-15.0). The objective response rate to enfortumab vedotin was 54%:
The median progression-free survival from the start of enfortumab vedotin was 7.0 months (95%CI 5.8-13.3), and median overall survival was 13.3 months (95%CI 10.8-NR):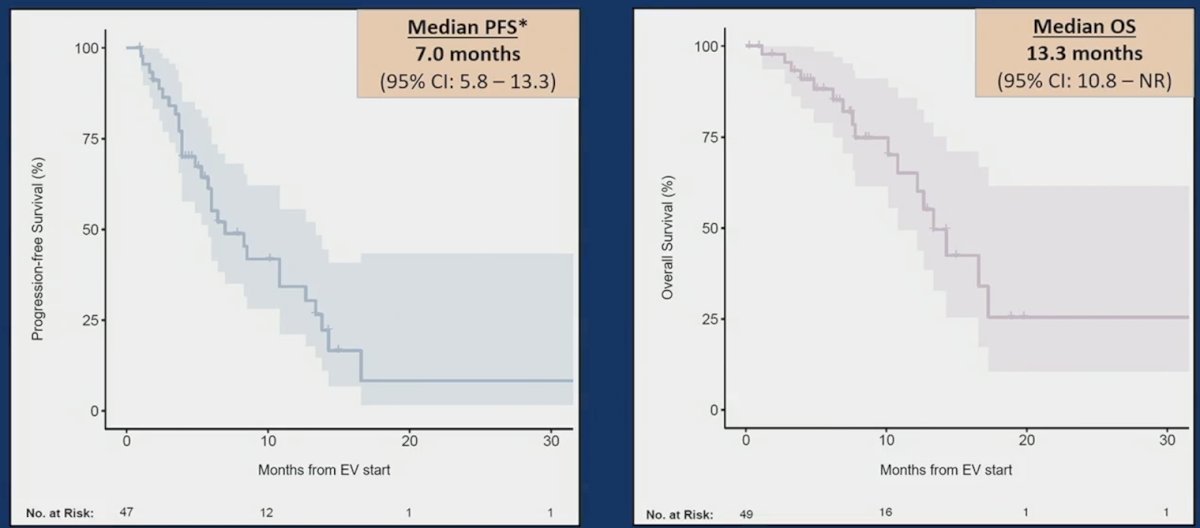
The median progression-free survival 2 measured from platinum-based therapy start until progression after starting enfortumab vedotin or death was 17.5 months (95% CI 15.2-22.5). Furthermore, the median overall survival from platinum-based therapy start was 22.5 months (95% CI 18.6-NR):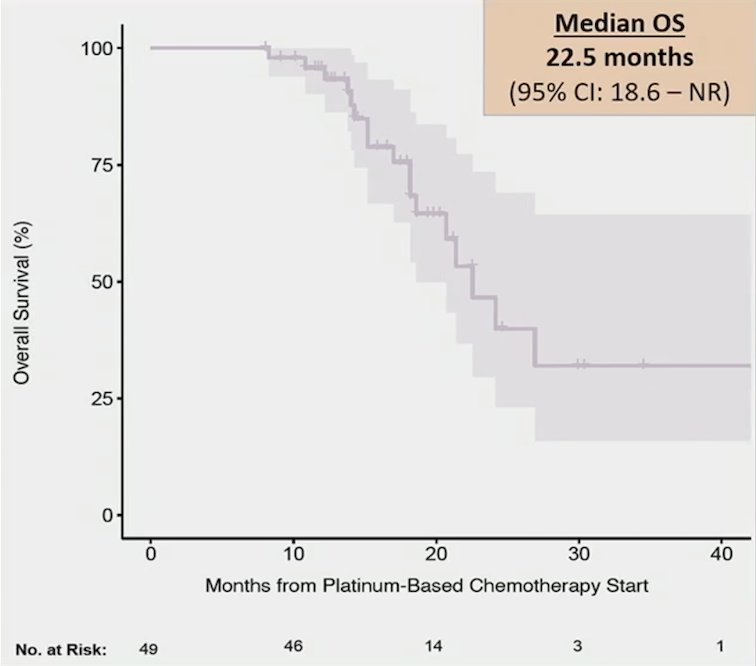
Outcomes did not differ among subgroups, except for improved progression-free survival and overall survival in patients with Bellmunt score 0-1 vs Bellmunt score 2-3: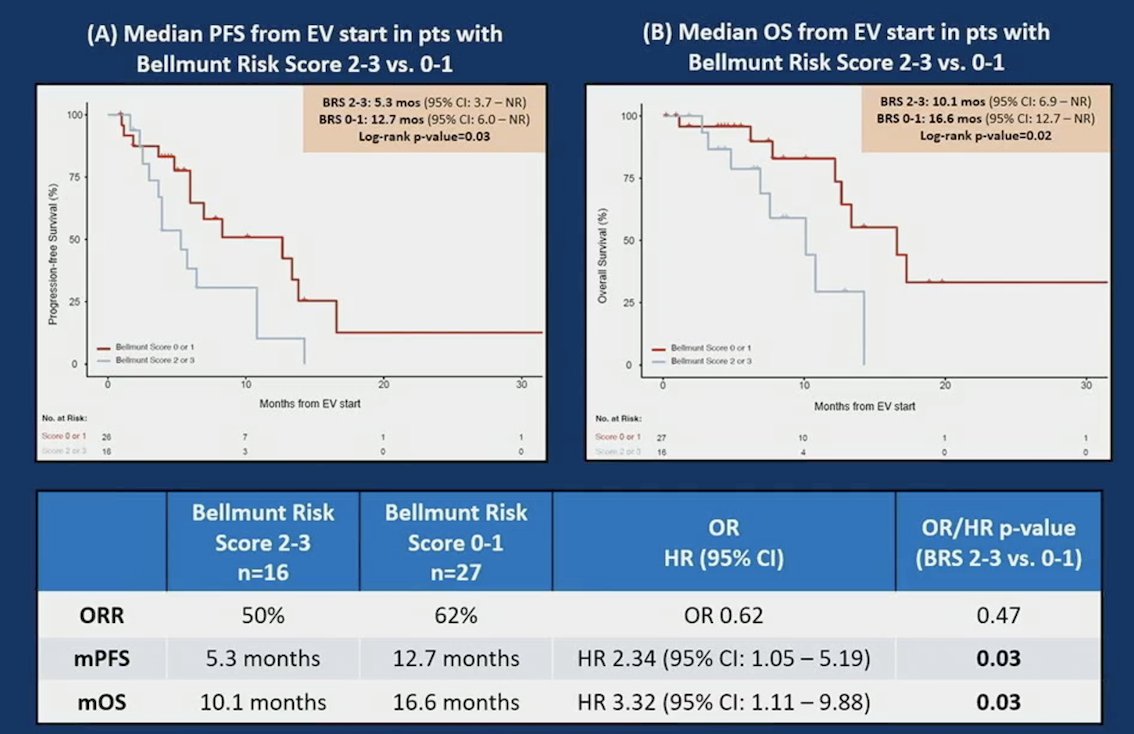
Of note, 29% of patients remained on enfortumab vedotin at the time of data cutoff, and 43% received subsequent therapy after enfortumab vedotin with a median time to next therapy of 6.4 months (95% CI 1.8-15.9). The reasons for discontinuing therapy and the next lines of treatment are as follows: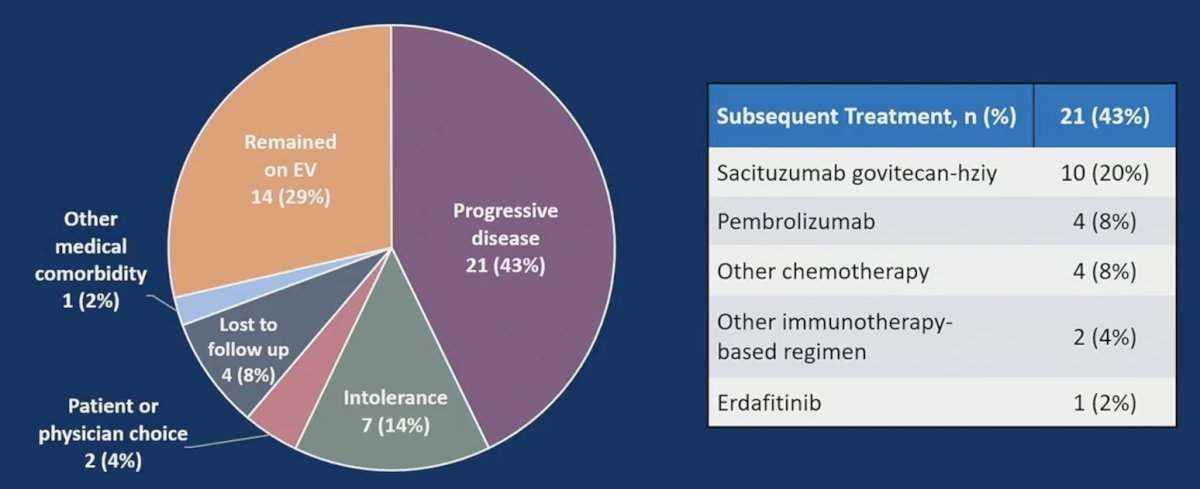
Limitations of this study include the retrospective design, selection/confounding biases, lack of central review of imaging, and the limited sample size.
Dr. Nizam concluded her presentation discussing outcomes in patients with advanced urothelial carcinoma treated with enfortumab vedotin after switch maintenance avelumab in the UNITE study with the following take-home points:
- Patients with advanced urothelial carcinoma treated with enfortumab vedotin after maintenance avelumab had outcomes consistent with data for enfortumab vedotin in platinum-based therapy- and checkpoint inhibitor-refractory advanced urothelial carcinoma
- Patients with higher Bellmunt scores had worse outcomes with enfortumab vedotin
- Identifying biomarkers associated with response and resistance to platinum-based chemotherapy and enfortumab vedotin are critical
- These data support the use of enfortumab vedotin as third-line therapy after progression on maintenance avelumab but should be validated in larger cohorts
Presented by: Amanda Nizam, MD, Cleveland Clinic, Cleveland, OH
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the Genitourinary (GU) American Society of Clinical Oncology (ASCO) Annual Meeting, San Francisco, CA, Thurs, Jan 25 – Sat, Jan 27, 2024.
Related content: Outcomes of Enfortumab Vedotin After Platinum and Avelumab in Bladder Cancer - Amanda Nizam
References:
- Powles T, Rosenberg JE, Sonpavde GP, et al. Enfortumab Vedotin in Previously Treated Advanced Urothelial Carcinoma. N Engl J Med 2021 Mar 25;384(12):1125-1135.
- Powles T, Park SH, Voog E, et al. Avelumab Maintenance Therapy for Advanced or Metastatic Urothelial Carcinoma. N Engl J Med 2020 Sept 24;383(13):1218-1230.
- Koshkin VS, Henderson N, James M, et al. Efficacy of enfortumab vedotin in advanced urothelial cancer: Analysis from the Urothelial Cancer Network to Investigate Therapeutic Experiences (UNITE) study. Cancer 2022 Mar 15;128(6):1194-1205.


