(UroToday.com) The 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) cancers symposium held in San Francisco, CA between January 25th and 27th was host to a urothelial carcinoma poster session. Dr. Philippe Barthelemy presented updated results from AVENANCE, a real-world study of the efficacy of avelumab first-line maintenance in patients with advanced urothelial carcinoma, as well as an analysis of subsequent treatments received.
In the JAVELIN Bladder I00 phase 3 trial (NCT02603432). avelumab 1st line maintenance + best supportive care significantly prolonged overall and progression-free survivals compared to best supportive care alone in patients with advanced urothelial carcinoma that had not progressed with 1st line platinum-based chemotherapy.1,2 At ≥2 years follow-up, the median overall survival improved from 15 months in the control arm to 24 months with addition of avelumab (HR: 0.76, 95% CI: 0.63 to 0.91, p=0.004). Based on these results, avelumab 1st line maintenance is now considered standard of care treatment for such patients, per numerous international guidelines.
Notably, in this trial, overall survival durations in the JAVELIN Bladder 100 trial were achieved despite only nine patients receiving enfortumab vedotin as 2nd line treatment after discontinuing avelumab 1st line maintenance (2.9% of discontinuing patients), reflecting the limited available options when the study was conducted. Given the evolving treatment landscape, studies to assess overall survival with different treatment sequences in patients with advanced urothelial carcinoma are needed. AVENANCE is an ongoing, real-world study evaluating the safety and effectiveness of avelumab 1st line maintenance in patients with advanced urothelial carcinoma in France. In this report, Dr. Barthelemy and colleagues presented updated data from AVENANCE plus additional analyses by subsequent 2nd line treatment, as well as exploratory analyses of overall survival measured from the start of 1st line platinum-based chemotherapy in this study population of patients without disease progression.
AVENANCE (NCT04822350) is an ongoing, multicenter, ambispective, non-interventional study. Eligible patients had locally advanced or metastatic urothelial carcinoma that had not progressed with 1st line platinum-based chemotherapy (i.e., ongoing complete response, partial response, or stable disease) and previous, ongoing, or planned avelumab 1st line maintenance treatment. Data collection started on July 13, 2021. No study-specific visits were required, and patients were assessed and followed as per standard clinical practice.
The primary endpoint was overall survival, measured from the start of avelumab treatment, with secondary endpoints including:
- Progression-free survival
- Duration of treatment
- Safety
An exploratory analysis of overall survival from start of 1st line chemotherapy was also performed, which excluded patients whose start date for chemotherapy was not provided or was recorded as being on or after the date of avelumab initiation.
Additionally, in this report, detailed analyses were performed in the overall population and in subgroups defined by 2nd line treatment received after discontinuing avelumab 1st line maintenance. Patients may have received 2nd line treatment within standard clinical practice, early access programs, or clinical trials.
At data cutoff (December 7, 2023), the median follow-up from avelumab intiation in the effectiveness population was 26.3 months (n=595). 21% of patients were still receiving avelumab treatment. Reasons for avelumab discontinuation included disease progression (73%), adverse events (11%), and death (9%). The median duration of avelumab treatment was 5.6 months.
330 patients received 2nd line treatment after avelumab (70% of patients who discontinued avelumab), which included chemotherapy (74%), an antibody-drug conjugate (19%), including enfortumab vedotin, and Sacituzumab Govitecan (2%). The baseline characteristics are summarized below:
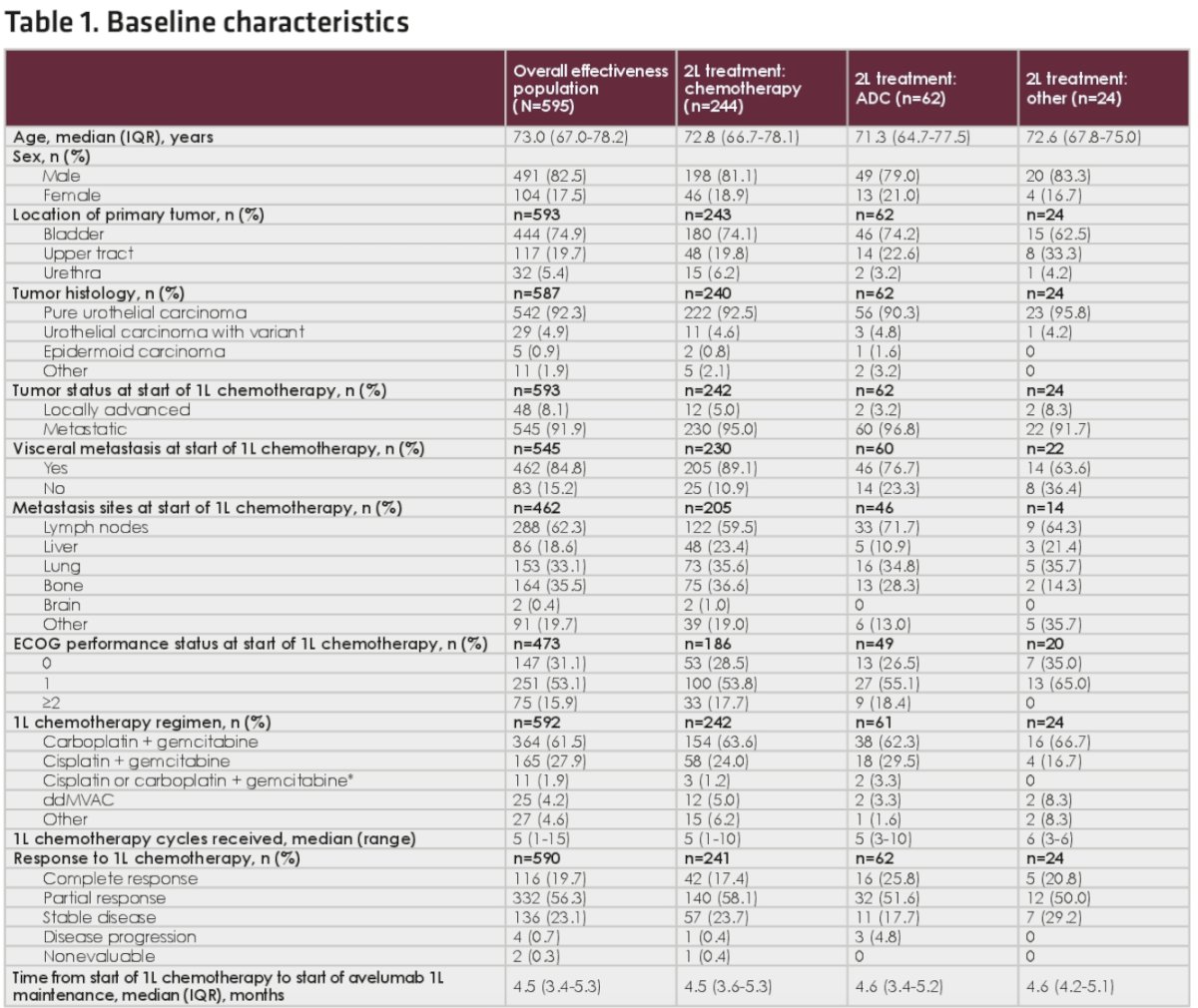
In the overall effectiveness population, the median overall survival from the start of avelumab 1st line maintenance treatment was 21.3 months. The 1- and 2-year overall survival rates were 67% and 46%, respectively.
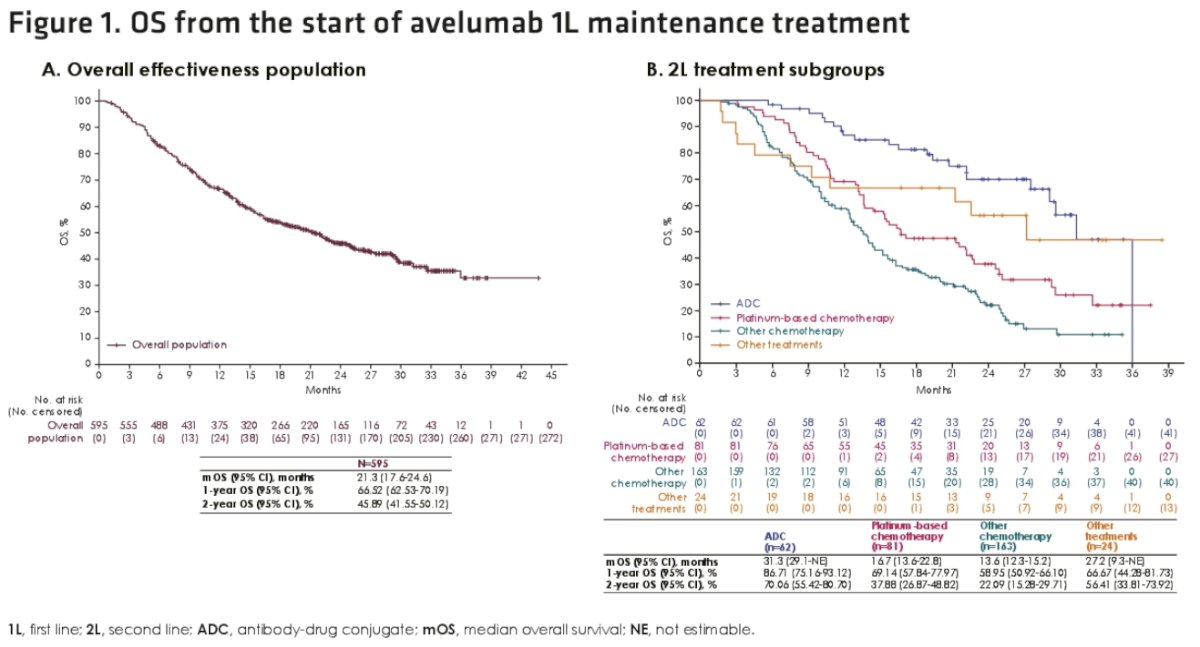
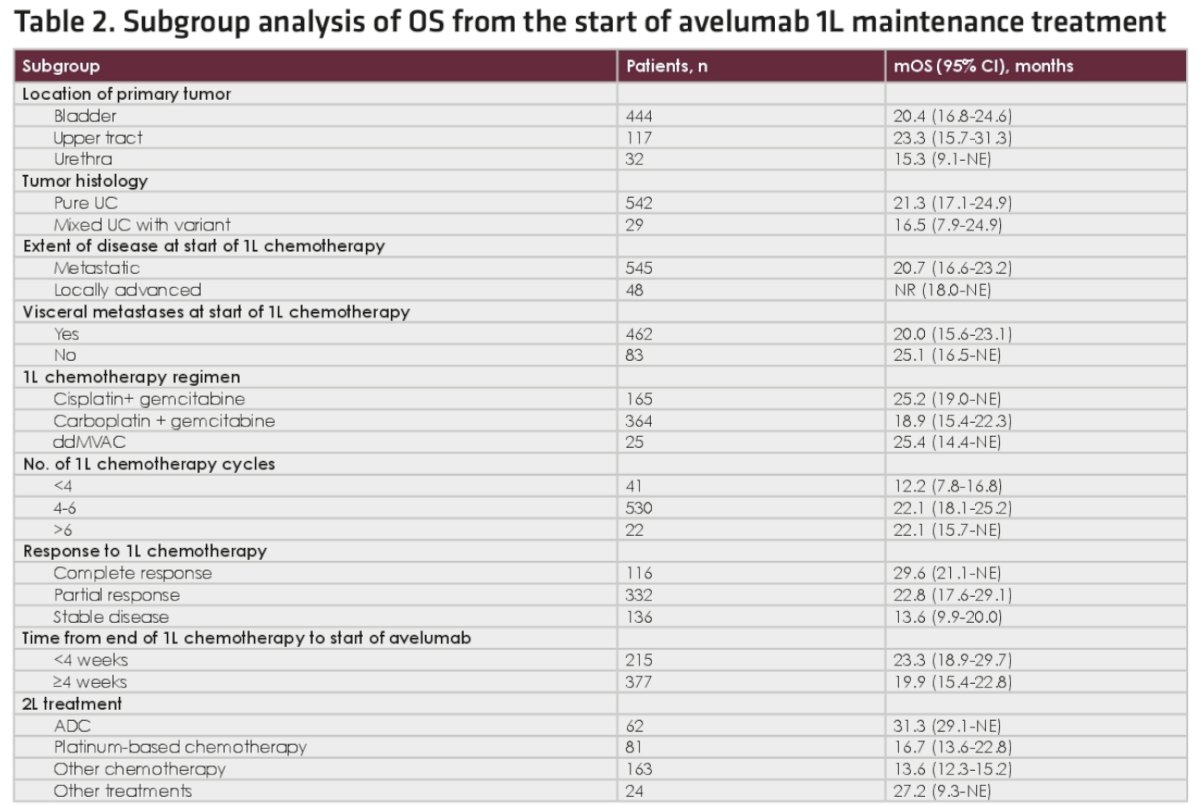
In patients who received 2nd line antibody-drug conjugate therapy after avelumab discontinuation, the median overall survival from start of avelumab was 31.3 months, with 1- and 2-year rates of 87% and 70%, respectively. The corresponding median overall survival for those receiving 2nd line chemotherapy were 14.4 months (platinum-based: 16.7 versus non-platinum-based: 13.6 months).
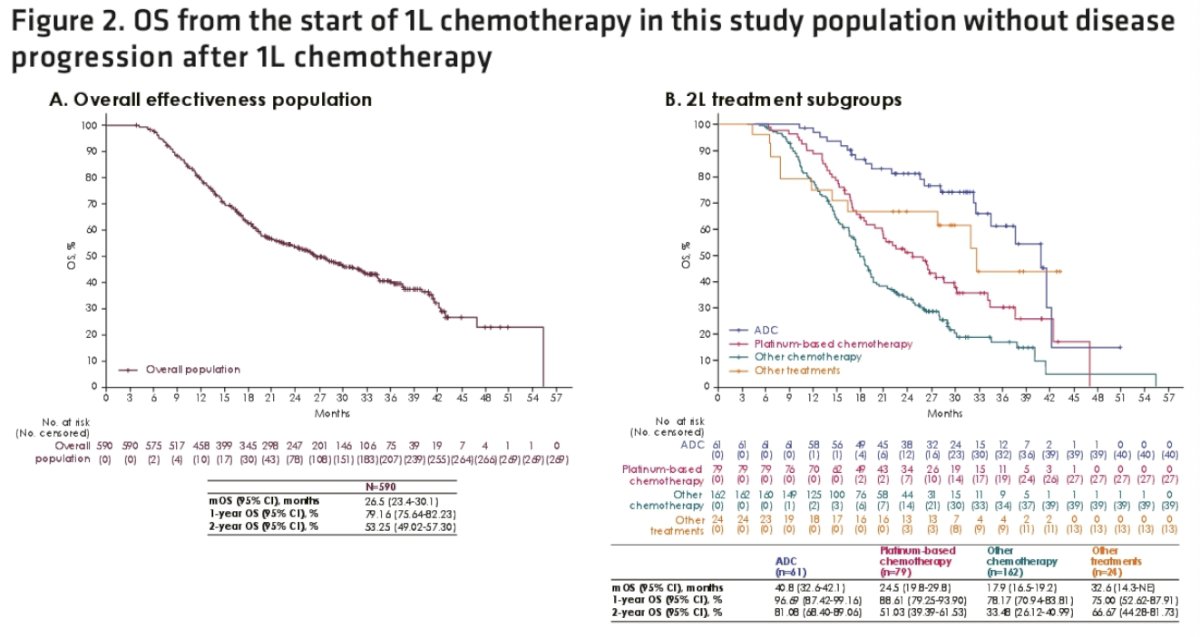
The median overall survival from the start of 1st line chemotherapy in this population without disease progression after 1st line chemotherapy was 26.5 months. The median overall survival from start of 1st line chemotherapy was 41 months for those who received 2nd line antibody-drug conjugates and 24.5 months for those receiving 2nd line platinum-based chemotherapy.
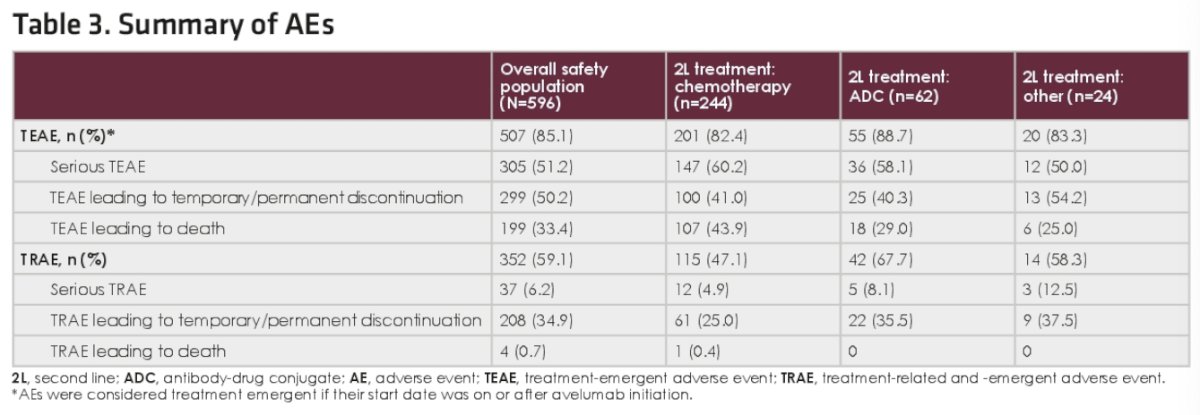
The safety/adverse events are summarized below:
Dr. Barthelemy concluded that:
- Longer-term follow-up of AVENANCE confirms the effectiveness and safety of avelumab 1st line maintenance treatment in a real-world setting
- The median overall survival from the start of avelumab 1st line maintenance was 21.3 months
- In subgroup analyses, median overall survival from the start of avelumab 1st line maintenance was 31.3 months in patients who received 2nd line antibody-drug conjugate treatment (mostly enfortumab vedotin) after avelumab 1st line maintenance, and 16.7 months in patients who received 2nd line platinum-based chemotherapy.
- In an exploratory analysis, median overall survival measured from the start of 1st line platinum-based chemotherapy in this population without disease progression was 26.5 months
- Median overall survival from the start of 1st line platinum-based chemotherapy was 40.8 months in patients who received 2nd line antibody-drug conjugate treatment after avelumab 1st line maintenance, and 24.5 months in patients who received 2nd line platinum-based chemotherapy
- At data cutoff, 21% of patients were still receiving avelumab 1st line maintenance treatment, and 55.5% had received 2nd line treatment
- Overall. these results support the recommendation of avelumab 1st line maintenance as standard of care in patients with advanced urothelial carcinoma that has not progressed with 1L platinum-based chemotherapy
- Results from exploratory analyses suggest that patients who receive 1st line platinum-based chemotherapy without disease progression followed by avelumab 1st line maintenance and 2nd line treatment with an antibody-drug conjugate, such as enfortumab vedotin, may have a median overall survival >3 years.
Presented by: Philippe Barthelemy, MD, PhD, Medical Oncology, Institut de Cancérologie Strasbourg Europe, Strasbourg, France
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, San Francisco, CA, January 25th – January 27th, 2024
References:
- Powles T, Park SH, Voog E, et al. Avelumab Maintenance Therapy for Advanced or Metastatic Urothelial Carcinoma. N Engl J Med 2020 Sept 24;383(13):1218-1230.
- Powles T, Park SH, Caserta C, et al. Avelumab First-Line Maintenance for Advanced Urothelial Carcinoma: Results From the JAVELIN Bladder 100 Trial After >/=2 Years of Follow-Up. J Clin Oncol. 2023;41: 3486-3492.


