(UroToday.com) The 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) cancers symposium featured a urothelial carcinoma rapid oral abstract session, including a presentation by Dr. Matthew Galsky discussing the interim analysis of ANTICIPATE phase II trial assessing oral APL-1202 in combination with tislelizumab as neoadjuvant therapy in patients with muscle-invasive bladder cancer. APL-1202 (nitroxoline) is a reversible and orally available MetAP2 inhibitor with anti-angiogenic and anti-tumor activities. Previously it has been shown that single-agent neoadjuvant PD-1 antibodies achieve pathological complete responses in a subset of patients with muscle-invasive bladder cancer. APL-1202 and PD-1 antibody combination therapy demonstrates synergistic effects in several model systems of cancer, including bladder cancer. Dr. Galsky and colleagues hypothesized that APL-1202, in combination with tislelizumab, a humanized IgG4 anti-PD-1 antibody, may be an effective neoadjuvant therapy in muscle-invasive bladder cancer.
This is a prospective multicenter randomized phase II trial for patients with newly diagnosed muscle-invasive bladder cancer for whom radical cystectomy is planned, and who are cisplatin ineligible or refuse cisplatin based neoadjuvant chemotherapy. Eligible patients were randomly assigned to Group 1 (APL-1202 + tislelizumab) or Group 2 (tislelizumab alone), with randomization stratified by PD-L1 expression. Neoadjuvant treatment is administered every 3 weeks for 3 cycles. The trial design for ANTICIPATE is as follows:
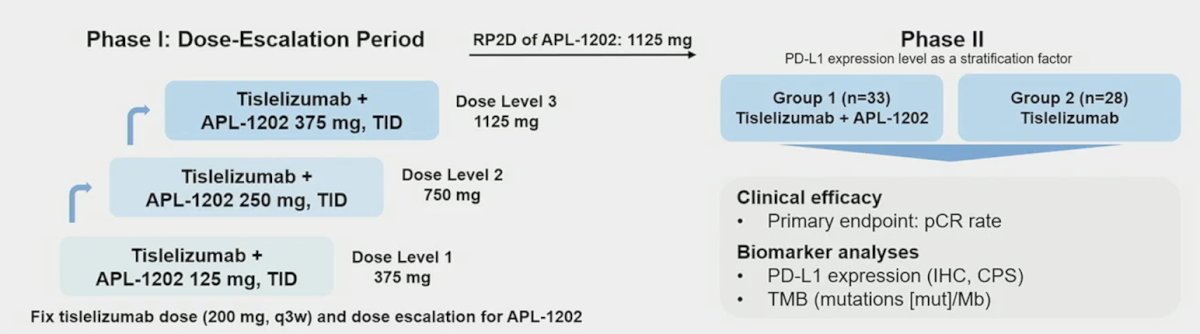
The primary endpoint is pathological complete response (pT0N0) rate. A Simon’s 2-stage design was employed with planned interim analyses after 18 evaluable patients in Group 1 and 14 evaluable patients in Group 2.
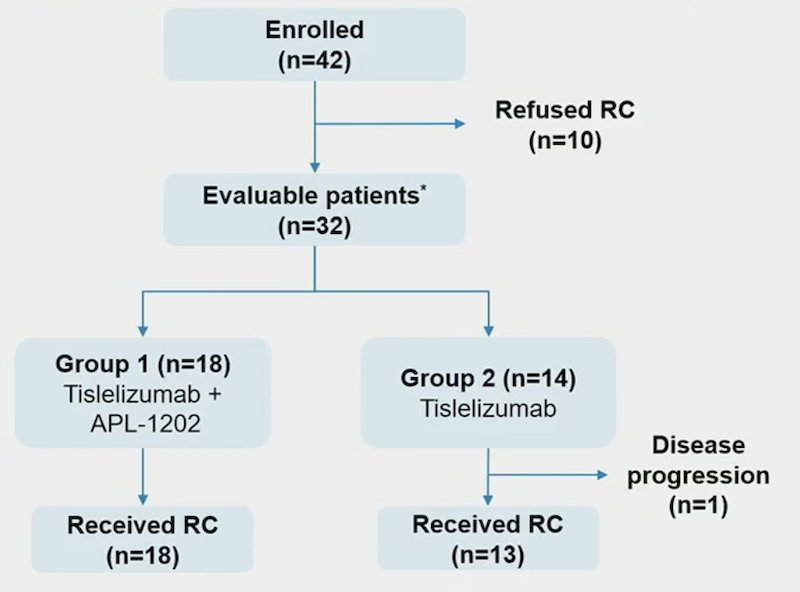
There were 42 patients enrolled and results for 32 evaluable patients for stage 1 of the 2-stage design are reported. Radical cystectomy was completed in 18/18 patients in Group 1 and 13/14 patients in Group 2, although there was one patient who could not undergo surgery due to disease progression and 10 patients refused radical cystectomy. The patient disposition is below:
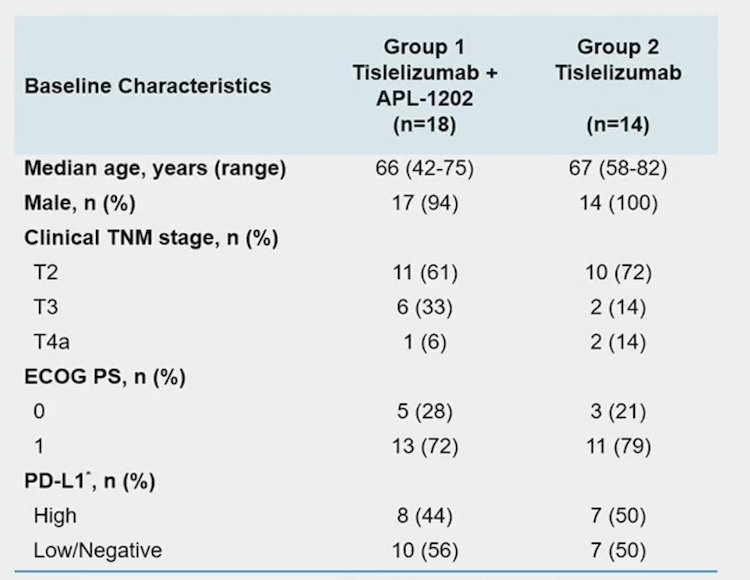
The 32 evaluable patients consisted of 11/18 (61%) and 10/14 (72%) cT2, and 6/18 (33%) and 2/14 (14%) cT3, and 1/18 (6%) and 2/14 (14%) cT4a in Group 1 and Group 2, respectively. PD-L1 expression was assessed using the VENTANA PD-L1 (SP263) Assay, including 8/18 (44%) patients in Group 1 and 7/14 (50%) in Group 2 was positive:
The pathologic complete response rate in Group 1 was 39% versus 21% in Group 2, whereas the pathologic downstaging rate (<pT2N0) was 44% for Group 1 and 21% for Group 2. The pathologic complete response rates in both Group 1 and Group 2 exceeded thresholds to trigger expansion to stage 2 of the 2-stage design. Specific to T3N0M0 patients, 2 (33%) of 6 patients achieved a pathologic complete response in Group 1, while no patient in Group 2 achieved a pathologic complete response:

Treatment emergent adverse events were reported in 17 (94.4%) patients in Group 1 and 11 (78.6%) in Group 2, with the most common (≥ 10%) treatment emergent adverse event of CTCAE grade ≥ 3 being anemia (n = 4, 22.2%), lymphocyte count decreased (n = 3, 16.7%) in group 1, and intestinal obstruction (n = 3, 21.4%) in group 2. Adverse events led to drug discontinuation in 3 (16.7%) patients in Group 1 (acute kidney injury, anemia, hepatic function abnormal) and 2 (14.3%) patients in Group 2 (immune hyperthyroidism, COVID-19), and no treatment-related adverse events led to death.
Dr. Galsky concluded his presentation by discussing the interim analysis of ANTICIPATE with the following take-home points:
- The pathologic complete response rates in both Group 1 (APL-1202 + tislelizumab) and Group 2 (tislelizumab) exceeded thresholds to trigger expansion to stage 2 of the 2-stage design
- APL-1202 + tislelizumab exhibited an acceptable safety profile
- The activity and safety of neoadjuvant APL-1202 + tislelizumab support further evaluation of this novel regimen
Presented by: Matthew Galsky, MD, Tisch Cancer Institute, Division of Hematology and Medical Oncology, Icahn School of Medicine at Mount Sinai, New York, NY
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, San Francisco, CA, January 25th – January 27th, 2024
Related content: ANTICIPATE II Trial: APL-1202 and Tislelizumab for Neoadjuvant Bladder Cancer Treatment - Matthew Galsky


