(UroToday.com) The 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) cancers symposium held in San Francisco, CA between January 25th and 27th was host to a urothelial carcinoma poster session. Dr. Petros Grivas presented the long-term patient-reported outcomes from the phase 3 JAVELIN Bladder 100 trial of avelumab 1st line maintenance for patients with advanced urothelial carcinoma (aUC).
In the JAVELIN Bladder 100 phase 3 trial, avelumab 1st line maintenance + best supportive care (BSC) significantly prolonged overall survival (OS) and progression-free survival (PFS), compared with BSC alone, in patients with aUC that had not progressed with 1st line platinum-based induction chemotherapy, with a median OS improvement from 15 to 24 months (HR: 0.76, 95% CI: 0.63 to 0.91, p=0.004).1,2 Based on results from JAVELIN Bladder 100, avelumab 1st line maintenance has been approved in multiple countries worldwide and is currently recommended as a standard of care by international guidelines.
In an ad hoc analysis, treatment with avelumab 1st line maintenance + BSC resulted in a consistently longer Q-TWiST, compared to BSC alone, indicating a net benefit in quality survival or well-being with avelumab. Initial analyses of patient-reported outcomes (PROs) in JAVELIN Bladder 100 showed that avelumab 1st line maintenance treatment resulted in stable health-related quality of life. In this analysis, Dr. Grivas and colleagues reported PROs data with long-term follow-up in the overall avelumab + BSC arm (any treatment duration) and in a subgroup with ≥12 months of avelumab treatment.
In the JAVELIN Bladder 100 trial, PROs were a secondary endpoint and were assessed at baseline, on day 1 of each 4-week cycle, at the end of treatment or withdrawal from the study, and up to 90 days post-treatment. The PRO instruments used were the NCCN/FACT Bladder Symptom Index-18 (NFBISI-18) and EuroQol EQ-5D-5L. Given the repeated measurements nature of the analysis within the same individuals followed longitudinally, analyses were conducted using mixed-effects modeling.
PROs were examined in all patients who received treatment in the avelumab + BSC arm for any duration and in a subgroup who had received ≥12 months of avelumab treatment. Of note, data were not evaluated in the BSC alone arm because few patients remained on study treatment at later time points, and comparative assessment was deemed not appropriate due to this attrition bias.
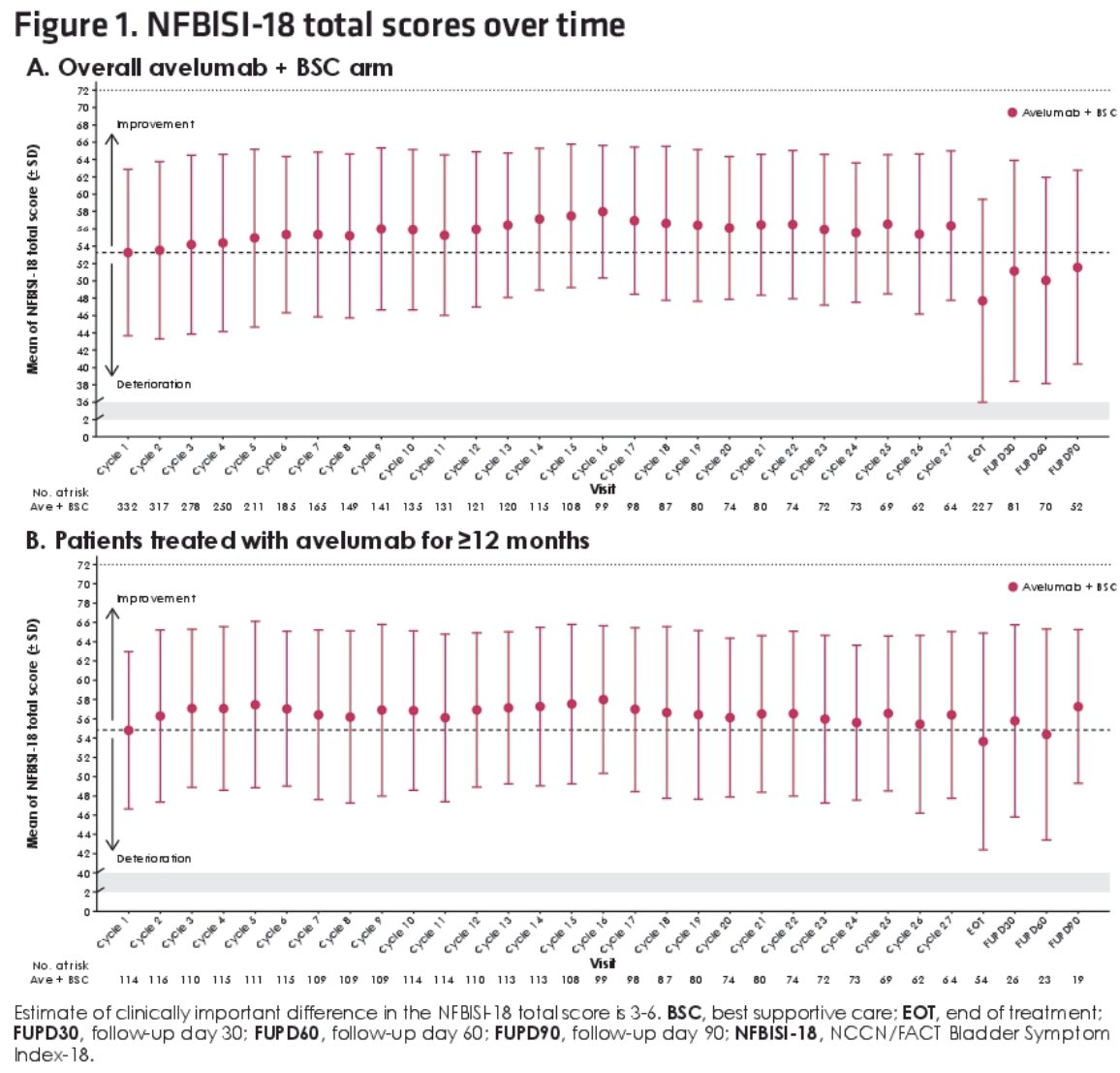
At data cutoff (June 4, 2021), median follow-up in the avelumab + BSC arm (n=350) was 38 months (≥2 years in all patients), and median duration of treatment was 5.8 months. In patients treated for ≥12 months (n=118 [34%]]), baseline characteristics were similar to those of patients in the overall avelumab + BSC arm, except for a higher proportion with ECOG performance status 0 (70% versus 61%) and PD-L1+ tumors (61% versus 54%) and a lower proportion with visceral metastases (48% versus 55%).
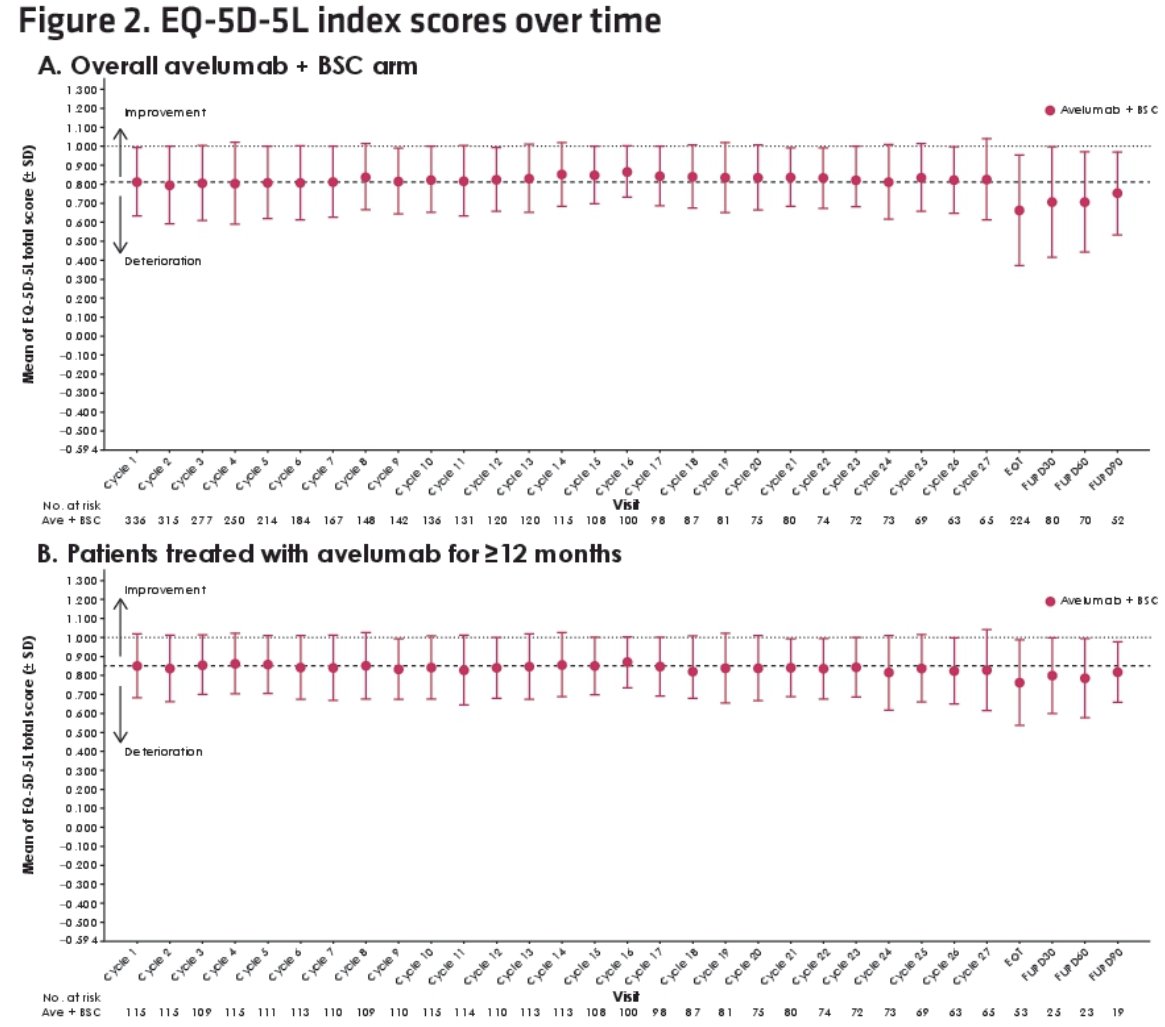
In both populations, completion rates for both PRO instruments among evaluable patients were >80% at all time points during treatment. On average, PRO scores remained stable throughout treatment, and no clinically important changes from baseline were reported.
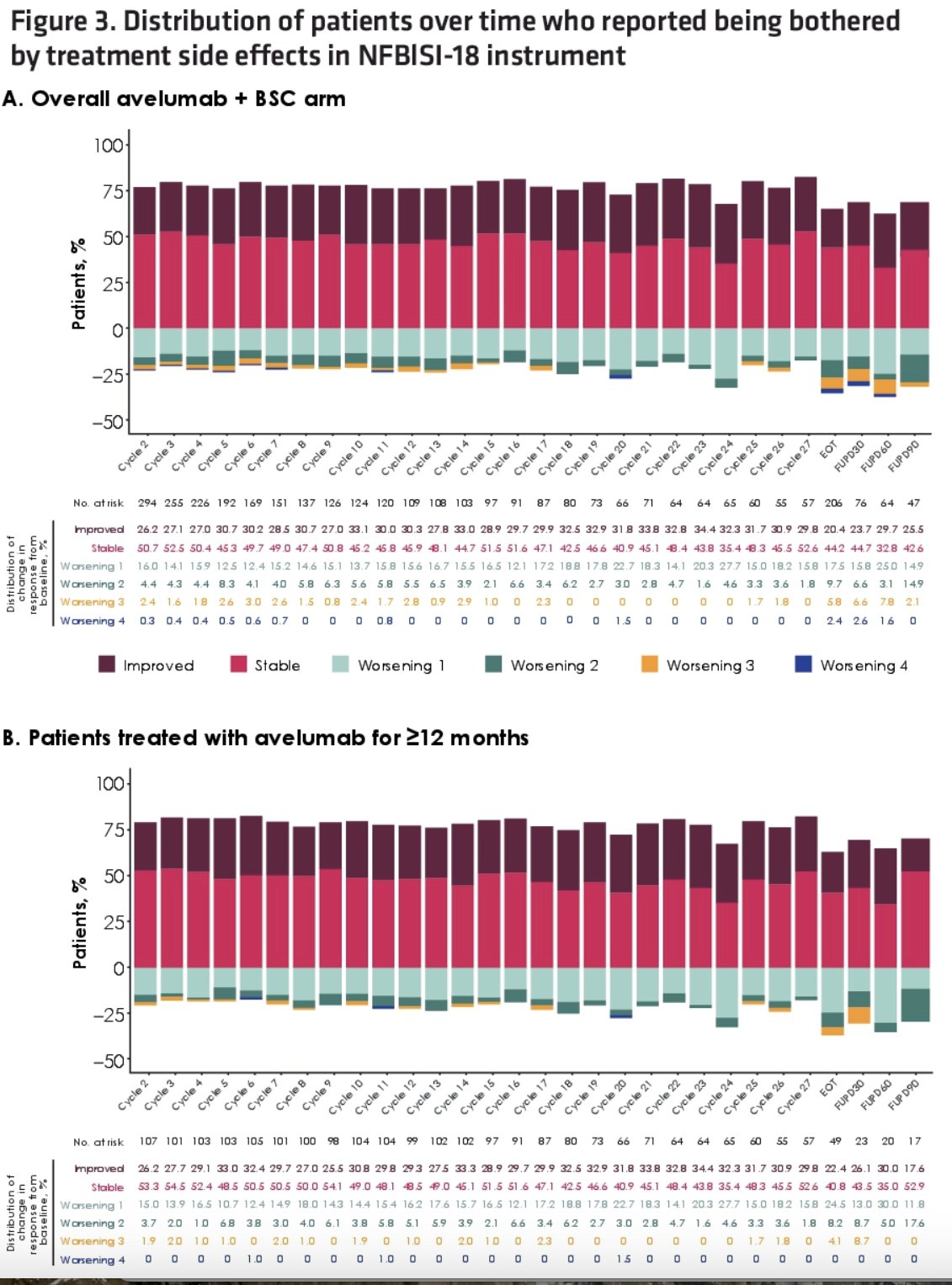
In the overall avelumab + BSC arm and in patients treated with avelumab for ≥12 months, approximately 75% of evaluable patients reported no change or a decrease in how much they were bothered by treatment side effects throughout 24 months of treatment.
Dr. Grivas concluded that:
- Long-term and exploratory analyses of patient-reported outcomes in patients with advanced urothelial carcinoma who received avelumab first-line switch maintenance + best supportive care in the JAVELIN Bladder 100 trial showed that prolonged avelumab treatment, including among patients treated for ≥12 months, was associated with stable PROs, indicating preservation of health-related quality of life
- These results complement previously reported results that compared PROs between study arms and ad hoc analyses demonstrating the acceptable long-term safety profile of avelumab 1st line maintenance, including in patients treated for ≥12 months
- These results are also consistent with a previous analysis showing that patients treated with avelumab 1st line maintenance + best supportive care had a consistently longer quality-adjusted time without symptoms of disease or toxicity (Q-TWiST) compared to patients who received BSC alone, reflecting the safety profile of avelumab 1st line maintenance in the context of an overall survival (OS) benefit.
- Overall, these data suggest that patients receiving long-term avelumab treatment may have preserved health-related quality of life and control of cancer-related symptoms with manageable treatment-related toxicity
- PRO results from this trial further support the use of avelumab 1st line maintenance until progression or unacceptable toxicity as standard of care in patients with advanced urothelial carcinoma who are progression-free after platinum-based chemotherapy
Presented by: Petros Grivas, MD, PhD, Professor, Division of Hematology & Oncology, University of Washington & Fred Hutchinson Cancer Center, Seattle, WA
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, San Francisco, CA, January 25th – January 27th, 2024
References:
- Powles T, Park SH, Voog E, et al. Avelumab Maintenance Therapy for Advanced or Metastatic Urothelial Carcinoma. N Engl J Med 2020 Sept 24;383(13):1218-1230.
- Powles T, Park SH, Caserta C, et al. Avelumab First-Line Maintenance for Advanced Urothelial Carcinoma: Results From the JAVELIN Bladder 100 Trial After >/=2 Years of Follow-Up. J Clin Oncol. 2023;41: 3486-3492.


